Official Journals By StatPerson Publication
|
Table of Content Volume 8 Issue 3 - December 2018
Comparison of dexmeditomidine and magnesium sulfate as adjuvants to lidocaine for intravenous regional anaesthesia for prolongation of duration of analgesia
Basappa M Diwanmal1, Prashant A Lomate2*
1Assistant Professor, 2 Associate Professor, Department of Anaesthesiology, Bharati Vidyapeeth Deemed (to be) University Medical College and Hospital, Sangli, Maharashtra, INDIA. Email: drprashantlomate@gmail.com
Abstract Background and Objectives: Intravenous regional anaesthesia (IVRA) is a very effective block for forearm and hand surgeries, but its effectiveness is limited by its short duration of action. Dexmeditomidine and magnesium sulfate have found to prolong the action of local anaesthetics through a peripheral mechanism. Our study compares the efficacy of two different types of adjuvants to lidocaine when used in IVRA for prevention of tourniquet pain and to increase the duration of postoperative analgesia. Methods: This is a prospective, randomized, double-blinded study. Sixty patients scheduled for hand or forearm surgery were randomly divided into two groups, comprising 30 patients each. Group D received 40 ml of 0.5% lidocaine + 1 µg/kg of dexmeditomidine and Group M received 40 ml of 0.5% lidocaine + 2 ml of 50% magnesium sulfate through the cannula on operative arm. The onset and recovery times of sensory and motor blocks, time to tourniquet pain, postoperative VAS score, time to first rescue analgesic and haemodynaµg parameters were recorded. Results: There was no statistically significant difference observed between the two groups with respect to sensory and motor block onset and regression times as well as intraoperative analgesic requirements (P ≥ 0.05). However, dexmedetomidine showed more favourable haemodynaµg variables, increased tourniquet tolerance time and prolonged duration of postoperative analgesia as compared to magnesium sulfate (P ≤ 0.05). Conclusion: Dexmedetomidine is a better adjuvant to lidocaine than magnesium sulfate for IVRA with respect to tourniquet tolerance, postoperative analgesia and haemodynaµg stability in the postoperative period. Key Word: Dexmeditomidine, intravenous, lidocaine, magnesium sulfate, motor block, sensory block.
INTRODUCTION Intravenous regional anaesthesia (IVRA) is a simple, safe and reliable technique of anaesthesia for forearm and hand surgeries. IVRA is also known as Bier’s block. It provides excellent anaesthesia and postoperative analgesia1 But this technique is limited by tourniquet pain and lesser duration of postoperative analgesia. To improve the quality of this block and postoperative analgesia various adjuvants to lidocaine have been tried with varying results.2,3 Alpha-2 agonists have gained popularity due to their sedative, analgesic and central sympatholytic properties. Dexmeditomidine is the new alpha-2 agonist having eight times more affinity for alpha-2 adrenoreceptors than clonidine. It reduces norepinephrine release at neuroeffector junction, inhibits neurotransmission of sympathetic nerves and decreases plasma catecholamines.4 Magnesium sulfate was also demonstrated as a good adjuvant for IVRA. This could be attributed to the antagonistic properties of magnesium on the NMDA receptors and its inhibitory properties for calcium channels. NMDA receptor antagonists can inhibit the induction of central sensitization owing to peripheral nociceptive stimulation and can eliminate hypersensitivity5,6 We have decided to compare the effects of dexmeditomidine and Magnesium sulfate when added to lidocaine during IVRA. We planned to investigate the onset and recovery times of sensory-motor blocks, quality of anaesthesia, haemodynaµg stability, intraoperative and postoperative pain and any side effects of the study drugs. Duration of tourniquet tolerance time and duration of postoperative analgesia were the primary aims of our study.
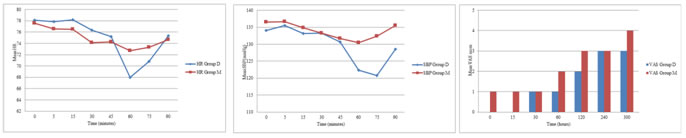
METHODS After obtaining the ethical committee approval, a randomized controlled study was formulated. Seventy patients scheduled for elective hand or forearm surgery expected to last for more than 30 min were enrolled in this study. Six patients were excluded on the basis of the exclusion criteria. Two patients, one from each group required conversion to general anaesthesia due to prolonged surgical duration and hence were excluded from the study. Of the remaining 60 patients, 30 were randomly assigned to the group D or group M. Thus the study population comprised 60 patients with ASA physical status I and II, aged 18- 60 years, scheduled for elective forearm and hand surgery (fractures of radius, ulna, metacarpal bones, carpal tunnel syndrome, ganglion excision etc). Patients with cardiovascular disease, epilepsy, hypertension, chronic obstructive pulmonary disease, morbid obesity, heart block, diabetes mellitus, renal disease, pregnancy, Raynold’s disease, sickle cell disease were excluded from the study. All patients were examined one day before surgery. Included patients received necessary information about the study and gave their written consents. The patients were randomly assigned to one of the two groups each containing 30 patients. These are: Group D and Group M. These groups were determined with closed envelopes. The subjects were blinded to the treatment they received. The anaesthesiologists who prepared and administered the medications were provided to be different. On arrival to operation theatre, routine monitoring was started. The patient’s baseline heart rate, systolic BP, diastolic BP, mean BP and oxygen saturation (SpO2) were recorded. Two intravenous cannulae were placed; one (22 G) on the dorsum of hand to be operated and other (20 G) on opposite hand for infusion of crystalloids. A double tourniquet was positioned on the operative arm. Then the operative extremity was exsanguinated with Esmarch bandage. The proximal tourniquet was inflated to 250 mmHg. Circulatory isolation of the arm was confirmed by the absence of radial pulse and loss of pulse oxymetry tracing. Group D received 40 ml of 0.5% lidocaine +1µg/kg of dexmeditomidine through the cannula on operative arm. Group M received 40 ml of 0.5% lidocaine + 2 ml of 50% magnesium sulfate through the cannula on operative arm. The solution was injected over 90 seconds by an anaesthesiologist blinded to the study drugs. The sensory block was assessed every 30 seconds after inflation of tourniquet by pin prick method. Sensory testing was done at thenar eminence for median nerve, hypothenar eminence for ulnar nerve and first web space for radial nerve. Sensory recovery was also assessed at these areas at 30 seconds intervals after deflation of torniquet. Motor function was assessed by asking the patient to flex and extend the wrist and fingers. Sensory block onset time was noted as the time from injection of study drug to the achievement of complete sensory block. Motor block onset time was the time from injection of study drug to complete motor block. After achievement of complete sensory and motor blocks, distal tourniquet was inflated to 250mmHg and proximal tourniquet deflated and patient handed over to surgeons for surgery. Mean arterial pressure (MAP), heart rate (HR) and SPO2 were recorded before and after tourniquet inflation, 5,10,15,30, 45,60,75 and 90 min after the injection of study drug. Hypotension (20% reduction from baseline) was treated with IV fluids and 6mg of inj. Ephedrine IV. Bradycardia (HR < 55/min) was treated with inj. Atropine 0.6 mg IV. The visual analog scale (VAS) was used to evaluate tourniquet pain at the intervals of 5, 10, 15, 25, 35 and 45 min after tourniquet inflation. VAS score: 0 = no pain and 10 = worst pain imaginable. For tourniquet pain 50 µg fentanyl was given IV for VAS>3. Time to first tourniquet pain was noted. It was measured before and after tourniquet application, 5, 10, 15, 20 and 40 min after the injection of study drug. The tourniquet was not deflated before 40 min of drug injection and was not inflated for more than 90 min. At the end of surgery tourniquet was released by the cyclic deflation technique. Sensory and motor block recovery times were noted. Postoperatively Patient’s pain and sedation scores were noted zero min (just after tourniquet deflation), 15 min, 30 min, 60 min, 120 min, 240 min and 300 min after deflation. Degree of sedation was assessed by Ramsay sedation scale:7 1 = anxious and/or agitated. 2 = co-operative, oriented and tranqill. 3=responds to commands only. 4=exhibits brisk response to light glabellar tap or loud auditory stimulus. 5=exhibits sluggish response to light glabellar tap or loud auditory stimulus. 6 = exhibits no response. Patients were given inj diclofenac sodium 75 mg IM when their VAS was > 4. The duration of analgesia was the time between tourniquet deflation and first injection of diclofenac. At the end of surgery, the quality of anaesthesia was assessed according to the following numerical scale. 4= excellent (no complaint from patient), 3=good (minor complaint with no need of analgesics, 2= moderate (complaint which required analgesics), 1= unsuccessful (patients given general anaesthesia). MAP, HR and SPO2 were recorded 0 min (immediately after deflation of tourniquet), 5, 10, 15, 20, 30 and 40 min after tourniquet deflation. Local and systemic complications if any were recorded during the study period. The results obtained in this study were presented in tabulated manner and analysed using Microsoft Excel and SPSS software (version 10, 2010) for windows. The two groups were compared by using t test. The results were expressed as mean ± SD. P value <0.05 was regarded as statistically significant. RESULTS The two groups were not statistically different with respect to ASA status and demographic data [Table 1 and Fig. 1]. There were no statistically significant differences between the two groups in terms of tourniquet time, duration of surgery, sensory block onset time and motor block onset time, whereas the sensory and motor block recovery time was more prolonged in group D compared with group M, although not statistically significant [ P ≥ 0.05 ] [Table 2 ]. Tourniquet pain onset time was comparatively longer in group D than in group M [P <0.05]. Three patients in group M and two patients in group D required inj. Fentanyl intraoperatively. This difference was statistically insignificant. [P >0.05] [Table2]. Quality of anaesthesia was excellent in both the groups [Table 2]. There was no significant change in heart rate (HR) intraoperatively, but there was temporary decrease in HR in group D after release of tourniquet. The difference between two groups was statistically significant [p<0.05] [Fig. 2], though the fall in HR was not more than 20% of baseline value. There was temporary fall in Mean arterial pressure (MAP) in group D after release of tourniquet [p<0.05] [Fig. 3]. The fall in MAP was not more than 20% of baseline value. Time to first rescue analgesic (duration of analgesia) was significantly longer in group D than in group M [P <0.001]. Postoperative VAS was three or below three for five hours in group D, but it was above three but below five in group M [Fig. 4]. Both the group showed comparable low level of sedation as depicted by Ramsay sedation scale after deflation of tourniquet. The difference was statistically insignificant. [p>0.05] [Table 2].
Table 1: Demographic data
Values are presented as mean±SD, SD – standard deviation. No statistically significant difference between the two groups. Table 2: Block characteristics
Values are presented as mean ± SD. SD- standard deviation, *Suggests statistically significant difference (P<0.05) Figure 1: Consort flow diagram
Figure 2 Figure 3 Figure 4 Figure 2: Comparison of Heart rate (HR /min) between two groups; Figure 3: Comparison of mean systolic blood pressure (SBP mmHg) between two groups; Figure 4: Postoperative pain score ( VAS 0 – 10 )
DISCUSSION We have proved in our study that the addition of 1µg/kg dexmeditomidine to lidocaine for intravenous regional anaesthesia (IVRA) prolonged the initial time of tourniquet pain and the duration of postoperative analgesia as compared to 1gm magnesium sulfate, without causing any side effects. IVRA is a simple and effective technique of providing anaesthesia for upper limb surgeries. The IVRA is popular because of its ease of administration, rapid onset and rapid recovery, complete muscular relaxation and controllable extent of anaesthesia. This technique is useful in short surgical procedures. In this technique limbs are exsanguinated with an elastic bandage and blood is squeezed proximally towards the heart. Then Pneumatic tourniquets are inflated to occlude the blood vessels. Then the local anaesthetic, lidocaine is slowly injected intravenously into the exsanguinated limb. But in IVRA the durations of tourniquet tolerance and postoperative analgesia are short as compared with peripheral nerve blocks. For this purpose, various additives to local anaesthetics like tramadol, clonidine, fentanyl, ketorolac, neostigmine, nonsteroidal anti-inflammatory drugs (NSAIDs) etc have been tried but with varying results.8 In our study, we compared the efficacy of two established adjuvants to lidocaine in IVRA, dexmedetomidine andmagnesium sulfate. The α2 adrenoceptor agonists namely clonidine and dexmeditomidine have shown to improve block quality ofIVRA2,9. Dexmeditomidine causes hyperpolarization of noradrenergic neurons, leading to suppression of neuronal firing in the locus ceruleus and inhibition of norepinephrine release. This causes modulation of nociceptive neurotransmission and hence analgesia10,11 Magnesium sulfate was also used as an adjuvant for IVRA with good results. Magnesium sulfate is NMDA receptor and calcium channel antagonist. NMDA receptors have shown to inhibit the induction of central sensitization. Calcium channel blockers have shown antinociceptive effects in animals.12 A Esmaoglu et al13 found that addition of 1 μg/kg dexmedetomidine to lignocaine for IVRA improved quality of anaesthesia and decreased analgesic requirements. Tramer and Glynn12 used magnesium for the treatment of chronic limb pain in IVRA and shown improved quality of the block and increased overall success rate. In our study we also found improved tourniquet tolerance and significant prolongation of duration of postoperative analgesia in dexmeditomidine group than in magnesium sulfate group. But the difference in onset and duration of sensory and motor blocks was statistically insignificant between two groups. El-Tahawy M et al14 compared dexmedetomidine and magnesium sulfate as adjuvants for IVRA and found no significant difference in sensory onset time, motor onset time, quality of anesthesia and duration of postoperative analgesia. But they found improved tourniquet tolerance by dexmeditomidine than magnesium sulfate, although this difference was statistically insignificant. Tourniquet pain is a major disadvantage in IVRA. Its mechanism is still unclear despite the role of A fibers and unmyelinated C fibers.15 Clonidine has also shown to depress the nerve action potentials especially in C fibers. This mechanism improves the quality of the local anesthetic block.3 Dexmeditomidine causes fall in BP and HR due to its sympathetic blocking property. In our study we observed temporary fall in HR and MAP in group D after release of tourniquet. The α-2 agonists produce sedation probably by activation of α-2 adrenoreceptors in the locus coeruleus.16 Memis et al.17 observed no sedation intraoperatively and also postoperatively with the use of 0.5 µg/kg dexmeditomidine in IVRA. They also found that addition of dexmeditomidine to lidocaine in IVRA caused attenuation of tourniquet pain and reduced consumption of fentanyl. Our study results are consistent with this study. We also found low level of sedation in both groups, but difference between the two groups was statistically insignificant. At the dorsal root neurons, α –2 agonists also inhibit release of substance P in the nociceptive pathway. This explains their analgesic property. Thus perioperative dexmeditomidine decreases both intraoperative as well as postoperative opioid or non opioid analgesics requirements.18 We found no significant difference in intraoperative fentanyl consumption in both groups. El-Tahawy M et al.14 also found no significant difference in intraoperative fentanyl consumption after dexmeditomidine and magnesium sulfate in IVRA. In their studies Sahmeddini MA et al19 and Bansal P et al20 found that magnesium sulfate when used as an adjuvant in IVRA was effective in reducing postoperative pain intensity and has also increased duration of postoperative analgesia. There are some limitations of this study. First, the study population was small. Second, we failed to use control group and third, we did not measure plasma catecholamines levels to demonstrate effectiveness of the study drugs in decreasing sympathetic nervous system activity.
CONCLUSION From this study we conclude that dexmeditomidine is more effective adjuvant in IVRA than magnesium sulfate for prolongation of postoperative analgesia. Further studies are required regarding doses of drugs or use of different drug combinations to completely prevent tourniquet pain and to provide prolonged duration of postoperative analgesia.
REFERENCES
|
|
 Home
Home