Official Journals By StatPerson Publication
|
Table of Content Volume 8 Issue 3 - December 2018
A randomised control study comparing intravenous fentanyl vs nebulised fentanyl for postoperative pain relief in patients undergoing elective breast surgeries under general anesthesia
Bhavana T1, Henjarappa K S2*, Namrata Ranganath3
1Junior Resident, 2Associate Professor, Professor and HOD, Department of Anesthesiology, Kidwai Memorial Institute of Oncology MH Marigowda Road, Bangalore. 560027 INDIA. Email: bhavana.sarma@gmail.com
Abstract Background: Relief of post operative pain with minimal side effects is a major goal during postanesthesia care. Intravenous fentanyl has been the gold standard for post operative pain relief. Fentanyl is a mu opioid receptor agonist characterised by high potency, rapid onset, short duration of action, lipid solubility and an apparent absence of serious side effects normally associated with opioids. Aim: To compare analgesic efficacy of nebulised fentanyl with intravenous fentanyl for post operative pain relief in patients undergoing breast surgeries under general anesthesia. Objectives: 1. To assess the analgesic efficacy of nebulised fentanyl in comparison to IV fentanyl for post-operative pain relief after breast surgeries. 2. To study Incidence and severity of side effects of fentanyl such as sedation, postoperative nausea and vomiting, respiratory depression, if any. Methodology: After our Hospital Research and Ethics Committee approval and obtaining informed written consent 90 patients aged between 20 and 65 years, belonging to ASA physical status I or II who were scheduled for elective breast surgeries under general anesthesia were included in this randomized control study. The patients were randomly assigned into three groups of 30 patients each to receive either Intravenous fentanyl 1mcg/kg (group C), or nebulised Fentanyl 2mcg/kg (Group N1) and nebulised Fentanyl 3mcg/kg (group N2). Results: These patients in both groups were comparable with respect to demographic characteristics age, sex, height, weight, ASA grading. Heart rates, SBP, DBP, MAP, SPO2 at basal value, after intubation, after extubation, and in PACU were similar and comparable in groups C, N1, N2. VAS scores were comparable at 0 min in groups C, N1, N2. At 5,10,15 min VAS scores were lower in control group than group N1 and N2. (P value The mean VAS scores increased in control group after 45 min where as in group N1 scores increased after 75min and in group N2 after 90min. The incidence of PONV was higher in group C compared to groups N1, N2. Statistically significant difference was observed between PONV grade among the three groups (P=0.093) Incidence of respiratory depression was compared in groups C,N1,N2. The P value is 0.0326 which is statistically significant. Key Word: intravenous fentanyl, nebulised fentanyl.
INTRODUCTION IASP (International society for study of pain) defines pain as an “Unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage”.1,2,3 Galen described pain as "A complex multidimensional human perception. It is divine to allay pain".4 Most patients undergoing surgical procedures experience acute postoperative pain. But less than half patients report adequate postoperative pain relief.5,6 Early postoperative pain is the most common, dominating complaint and primary reason for the prolonged convalescence after most surgical procedures. Intense acute pain after surgical procedures might predict development of chronic pain.7 Postoperative pain is the most common clinical problem in hospitals among surgical patients and is one of the main reasons for overnight hospital stay in 17-41% of surgical day care patients. From the very beginning we have shifted through many modalities to control and relieve pain but with variable results. Despite improved understanding of pain and sophisticated medical technology, post operative pain remains a challenge for the anaesthesiologist.8,9 Acute postoperative pain management is not only a human feeling, but it is a key aspect of postoperative care.9 As acute pain, regardless of its site, can adversely affect nearly every organ function and so affects the postoperative morbidity and mortality. Prevention and treatment of postoperative pain continues to be a major challenge in post-operative care and plays an important role in early mobilization and well-being of the surgical patients.10-13 Effective postoperative analgesia results in improvement of respiration and stress on cardiovascular system, early return of GIT motility, early ambulation and discharge from hospital. Acute pain results in physiological and psychological responses in the patient, majority of which are detrimental to postoperative outcome. It therefore stands to reason that adequate relief of pain might contribute to better perioperative outcome.14,15,16 Breast surgery is one of the most common forms of surgery conducted in the hospitals. Even relatively minor breast surgeries are associated with significant postoperative pain.17 Poorly controlled postoperative pain has negative physiological and psychological consequences. Furthermore, effective acute pain control preserves immune function, both by suppressing the surgical stress response and by decreasing the need for general anaesthetics and opioids. Acute postoperative pain is an integral risk factor in the development of chronic post mastectomy pain. Acute postsurgical pain commonly exists after the breast cancer surgery. 54% of the patients who received breast cancer surgery experienced clinically meaningful pain (defined as worst pain intensity larger than or equal to 5 in 0-10 numerical rating scale).18 Systemic opioids have been the mainstay of pain management in the past and still continue to be a popular technique around which other strategies are built. Opioids are usually administered by intramuscular (IM), intravenous (IV), subcutaneous (SC) or transdermal routes. Common adverse effects of opioids are sedation, pruritus, nausea, vomiting, slowing of gastrointestinal function and urinary retention. Clinically, meaningful adverse effects are dose-related. Fentanyl is a strong opioid increasingly used in treating acute pain because of its lack of active metabolites and fast onset of action.19 Intravenous (IV) route for Fentanyl administration has been the gold standard for post-operative pain relief. However, it is often associated with complications such as respiratory depression, bradycardia and hypotension. Newer routes of Fentanyl administration as intranasal and inhalational were successfully utilized for relieving of acute pain. Pulmonary administration is a new promising non-invasive method for systemic Fentanyl. Fentanyl being highly lipophilic is suitable for use through this route. Further, it has been observed that on inhalation Fentanyl is absorbed rapidly and reaches maximum serum level in approximately 2min.20 Thus, the aim of this study was to compare the analgesic efficacy of nebulised Fentanyl with IV Fentanyl for post-operative pain relief in breast surgeries. MATERIAL AND METHODS Study Site: The patients are selected from pre-anaesthesia clinic of Kidwai Memorial Institute of Oncology, Bangalore. Study Population: 90 consecutive patients admitted for breast surgeries under General Anaesthesia at Kidwai Memorial Institute of Oncology during the period of 12/2015 to 05/2017 were selected for the study who qualified inclusion criteria after obtaining the institutional ethical committee approval. Inclusion Criteria:
Exclusion Criteria:
METHODOLOGY A total 90 patients were distributed into 3 groups of 30 patients each randomly by computer generated numbers.
Patients posted for breast surgeries and fulfilling the inclusion and exclusion criteria after undergoing preanaesthetic check-up were explained regarding the surgical procedure. A written informed consent was obtained from the patients willing to be a part of the study. All the patients were familiarized with pain scoring VAS scale. Premedication, induction and maintenance of anaesthesia were standardized. All patients were premedicated with Tab. Ranitidine 150mg and Tab. Alprazolam 0.5mg 12hr before surgery. After confirming NPO status, routine non-invasive monitoring with pulse oximetry, NIBP, ECG, was initiated in the operation theatre. Basal vitals were noted. Adequate intravenous access was secured in all patients, and subsequently premedicated with Inj. Glycopyrrolate (0.01mg/kg), Inj. Ondansetron (0.05mg/kg), Inj. Midazolam (0.05mg/kg), and Inj. Fentanyl (1.5mcg/kg). After preoxygenation for 3 min with 100% Oxygen, General Anaesthesia was induced with Inj. Propofol 2mg/kg and Inj. Succinylcholine (1.5mg/kg). Patients were intubated with appropriate cuffed Endotracheal Tube and tube position was confirmed and connected to volume controlled mode of mechanical ventilation. Anaesthesia was maintained with nitrous oxide 50%, O2 50% and isoflurane. For maintenance of relaxation Inj. Vecuronium bromide was given, an initial loading dose of 0.08 mg/kg followed by intermittent doses of Inj. Vecuronium (0.01mg/kg). Intraoperative monitoring consisted of NIBP, ECG, EtCO2 and SpO2. At the end of surgery, anaesthesia was reversed with Inj. Neostigmine (0.05mg/kg) and Inj. Glycopyrolate (0.01mg/kg). Patients were extubated after complete neuromuscular recovery in deep inspiration after thorough suctioning in fully awake state. After surgery patients were shifted to PACU, standard monitors were connected. Whenever the patient complained of pain or the VAS score reached >4, the patients received IV or nebulised Fentanyl accordingly. Group C received intravenous Fentanyl, 1 mcg/kg in 5ml normal saline solution and an equivalent volume of nebulised normal saline. Group N1 received nebulised Fentanyl 2mcg/kg in 5 ml Normal Saline solution and an equivalent volume of Normal Saline intravenously. Group N2 received nebulised Fentanyl 3mcg/kg in 5ml normal saline solution and equivalent volume of normal saline intravenously. Patients were nebulised by a standard ventimask with nebulisation chamber at a flow rate of oxygen 8-10 l/min for 8min. After completion of nebulisation, time of onset of analgesia was noted. Patients were observed initially at 5, 10, 15 minutes and then at an interval of 15 minutes upto 2 hrs. Patients who complain of pain even after 15 minutes were excluded from the study and were given alternative analgesia Inj. Paracetamol 15mg/kg intravenous infusion over 20min.
Parameters Noted:
Grade 0: No nausea and vomiting Grade 1: nausea without vomiting Grade 2: nausea with vomiting < 3 episodes Grade 3: nausea with vomiting > 3 episodes
Oxygen saturation < 90% without oxygen supplementation
Statistical Methods: Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented on Mean ± SD (Min-Max) and results on categorical measurements are presented in Number (%). Significance is assessed at 5% level of significance. Dependent variables were normally distributed. Samples drawn from the population were random. Cases of the samples were independent. Analysis of variance (ANOVA) has been used to find the significance of study parameters between three or more groups of patients. Chi-square/Fisher Exact test has been used to find the significance of study parameters on categorical scale between two or more groups, Non-parametric setting for Qualitative data analysis. Significant figures: +Suggestive significance (P value: 0.05<P<0.10) *Moderately significant (P value: 0.01<P £ 0.05) **Strongly significant (P value: P£0.01)Statistical software: The statistical software namely SPSS 18.0, and R environment ver.3.2.2 were used for the analysis of the data and Microsoft Word and Excel have been used to generate graphs, tables etc.
OBSERVATIONS AND RESULTS Ninety (90) patients in ASA grade I and II of female sex meeting the inclusion criteria, posted for elective breast surgery under general anaesthesia were selected for the study. The study was focussed to evaluate and compare the analgesic efficacy of Fentanyl given by intravenous route and as nebulisation in patients who underwent elective breast surgeries. The Study was A comparative three group study design. Age, Gender distribution, height, weight, ASA grading and baseline vitals heart rate, blood pressures, SP02 distribution in two groups of patients studied had no significant difference in mean. Table 1: Comparison of VAS Score in three groups of patients studied
ANOVA test
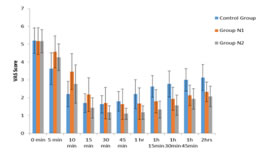
Figure 1: Comparison of VAS Score in three groups of patients studied *denotes significant difference VAS scores were recorded in groups C,N1,N2 at 0min, 5min, 10 min, 15 min, 30 min, 45min, 60 min, 75 min, 90 min, 105 min, 120 min and were statistically analyzed using ANOVA test. At 0 min VAS scores were similar and comparable between the three groups. There was no statistically significant difference in VAS scores measured between the groups at 0min (P=0.978). VAS scores were lower in group C at 5, 10, 15 min than group N1 and N2 and this difference is statistically significant with P values <0.001, <0.001, 0.001 respectively. At 30 min VAS score in group C was lower than N1 but higher than N2. At 45min VAS scores in group C were higher than both groups N1 and N2. VAS scores at 60min, 75min, 90min, 105 min and 120min were highest in group C compared to N1 and N2 making a statistically significant difference with P values < 0.001. At 5, 10, 15, 30, 45, 60, 75, 90,105 and 120 min VAS scores were lower in group N2 compared to group N1.
Table 2: Incidence of PONV in three groups of patients studied
P=0.093+, significant, Fisher Exact test
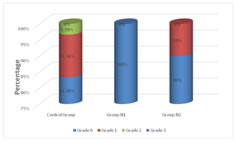
Graph 2: Incidence of PONV in three groups of patients studied Incidence of PONV was compared in groups C, N1, N2. None of the patients had PONV in group N1. Incidence of grade 0 in group N1 is 100%. In group C, incidence of grade 0 PONV was 83.3% and in group N2 it was 90%. Incidence of grade 1 was lowest in group N1 (0%) compared to N2 (10%) and C (13.3%). Incidence of grade 2 was lowest in N1 and N2 (0%) compared to group C (3.3%). Incidence of grade 3 PONV was 0% in the three groups. Statistically significant difference was observed between PONV grade among the three groups (P=0.093) showing that the incidence of PONV was higher in group C compared to groups N1, N2.
Table 3: Respiratory depression in three groups of patients studied
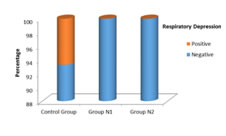
P=0.326, Significant, Fisher Exact test Figure 3: Respiratory depression in three groups of patients studied Incidence of respiratory depression was compared in groups C, N1, N2. It was observed in 2 patients in group C and the incidence was 6%, which was higher than group N1 (0%) and N2 (0%) in which no patients have experienced the symptoms. The P value is 0.0326 is statistically significant.
DISCUSSION In our study, VAS scores at 0 min in all the 3 groups were comparable with no statistical significant differences (p>0.05). At 5, 10, 15 min VAS scores were lower in group C than group N1 and N2 and this difference is statistically significant with P value <0.001. Worsley et al20 conducted a study comparing three groups of placebo, nebulised Fentanyl 100 mcg and nebulised Fentanyl 300 mcg for postoperative pain relief in patients who underwent various surgeries under general anaesthesia. Serum Fentanyl levels after inhalation of 100 mcg of nebulised Fentanyl reached a plateau around 0.04 ng/ml and after 300 mcg of nebulised Fentanyl at around 0.1 ng/ml after 15 minutes showing that peak concentrations of nebulised Fentanyl are reached after approximately 15 min. They concluded that useful analgesic effect was demonstrated despite low blood levels and the time to alternative analgesia was prolonged significantly in both nebulised Fentanyl groups which correlate with the findings of our study. In our study, the VAS scores after 45 min were higher in the group C than group N1 and N2. This difference is statistically significant with P values <0.001. The mean VAS scores increased in group C after 45 min. At 60 min, 75min, 90 min, 105min, 120 min VAS scores in group C were higher than N1 and N2 with statistically significant P value of < 0.001. In groups N1 and N2 VAS scores were higher compared to group C initially due to delayed onset of action of nebulised Fentanyl and continued to be low after 15 min. In group N1, scores increased after 75min and in group N2 after 90min. The duration of analgesia with Fentanyl nebulisation was prolonged than with intravenous Fentanyl. This could be due to slow rise in peak plasma concentration with nebulised Fentanyl compared to IV Fentanyl. Our findings correlate with the study conducted by Anil P Singh, Sritam S Jena, et al21 in 2013 on patients who underwent lower abdominal surgeries comparing analgesic efficacy of IV Fentanyl vs nebulised Fentanyl. 90 patients were divided into three groups of 30 each. In the post-operative care unit, at the time of first onset of pain (VAS score > 4) Fentanyl was administered either IV 2 µg/kg (group C) or by nebulisation of solution containing 3 µg/kg (group N1)or 4 µg/kg (group N2) Fentanyl over 8 min. Observations were made for pain relief by visual analogue scale score 0-10. They found that in the nebulisation group, the analgesic efficacy of Fentanyl was dose dependent with a delayed onset of analgesia 10 min vs. 5 min in the intravenous Fentanyl group. Nebulisation with 4 µg/kg Fentanyl produced analgesia at par to 2 µg/kg IV Fentanyl with prolonged duration (90 min vs. 30 min). They concluded that nebulised Fentanyl 4mcg/kg has slower onset of action (10 min vs. 5 min) and prolonged duration compared to IV Fentanyl 2mcg/kg (90 min vs. 30 min). Reza Ershad, Md Mozaffer Hossain et al22 compared the efficacy of nebulised Fentanyl 4mcg/kg with IV Fentanyl 2mcg/kg for postoperative pain relief after lower abdominal surgery. In the nebulisation group, it was observed that the analgesic efficacy of Fentanyl had delayed onset 10 min vs. 5 min in the intravenous group. Nebulisation with 4 µg/kg Fentanyl produced similar analgesic effects as IV Fentanyl 2 µg/kg with prolonged duration (90 min vs. 30 min). These findings are also correlating with our study. Salah Kamal et al23 in 2014 compared the nebulised Fentanyl as an alternative to the intravenous (IV) Fentanyl for analgesia after abdominal surgery in paediatric patients and they have observed that nebulised Fentanyl 2 µg/kg produced analgesia similar to IV Fentanyl 2 µg/kg but with delayed onset (5min vs 15min) and prolonged duration, these results are similar to our study. Similar results were observed in another study conducted by Saranya R, Anjali Modak (2016)24 in which they compared the analgesic efficacy of nebulised Fentanyl with IV Fentanyl for postoperative pain relief in lower abdominal surgery. In this study, it was found that the quality of analgesia after nebulisation with 4mcg/kg Fentanyl was effective, with delayed onset of action (10 minutes) and the duration of pain relief in the nebulisation group was prolonged when compared with the intravenous group (90minutes vs. 30minutes). We have also compared the incidence of PONV and respiratory depression in our study. The severity of PONV was graded on a four point ordinal scale where grade 0 – no nausea and vomiting, grade 1 – nausea without vomiting, grade 2–nausea with vomiting < 3 episodes, grade 3–nausea with vomiting>3 episodes. Respiratory depression was considered as respiratory frequency ≤ 8/minute or Oxygen saturation < 90% without oxygen supplementation. Group C had 83% patients with grade 0, 13.3% grade 1,3.3% grade 2 and 0% with grade 3 on PONV scale. In Group N1, incidence of grade 0 PONV was 100% and grade 1 and incidence of grade 2 and grade 3 PONV was 0%. In group N2, incidence of grade 0 PONV was 90% and grade 1 was 10% and grade 2 and grade 3 was 0% on PONV ordinal scale. Hence, the incidence of PONV is greater in with IV Fentanyl than nebulisation. The incidence of PONV in group C was higher than group N1 and N2 and it was higher in group N2 than N1and the difference is statistically significant with P value 0.093. In the study conducted by Anil P Singh, Sritam S Jena, et al21 they observed the incidence of side effects such as PONV, sedation, hypoxia, pruritus, urinary retention. Incidence of PONV in intravenous Fentanyl group was 13%. In nebulisation groups it was 4%. But this difference was not statistically significant in their study. However, in contrast in our study there was a statistically significant difference in PONV grading in intravenous Fentanyl compared to nebulised Fentanyl. In the study by Salah Kamal et al26 the incidence of PONV was 6.6% in nebulisation group where as it was 10% in IV Fentanyl group but this difference was not statistically significant. In a similar study by Saranya R, Anjali Modak23 they found that incidence of PONV in nebulisation group was lesser and delayed than the intravenous group. In our study, incidence of respiratory depression was also compared. In group C, it was 6% where as no symptoms of respiratory depression were recorded in groups N1 and N2. In the study by Anil P. Singh, Sritam S. Jena, et al21 in contrary to our study there was no documented respiratory depression in all the three groups. The incidence of respiratory depression in the study by Salah Kamal et al26 was 13.3% in intravenous group compared to 0% in nebulised Fentanyl group. Where as in our study the incidence of respiratory depression in intravenous Fentanyl group was 6% which was less when compared to the incidence in intravenous group of their study. Saranya R, Anjali Modak23 have found that the incidence of respiratory depression was lesser and delayed in nebulised Fentanyl group than IV Fentanyl group.
CONCLUSION From our study, we conclude that nebulised Fentanyl has comparable analgesic efficacy to intravenous Fentanyl with delayed onset and prolonged duration of action. The incidence of side effects like PONV and respiratory depression are less with nebulised Fentanyl when compared to intravenous Fentanyl. Hence, Nebulised Fentanyl is a novel promising modality of analgesia with longer duration of action and lesser side effects but with delayed onset and requires further studies to validate the results. REFERENCES
|
|
 Home
Home