Official Journals By StatPerson Publication
|
Table of Content - Volume 5 Issue 3 - March 2018
Study of oxidative stress and antioxidants among pre eclamptic pregnant women
Bhanupriya Tammineni1, Sowjanya Yerram2*
1Assistant Professor, Department Of Biochemistry, ASRAM Medical College, Eluru, West Godavari, Andhra Pradesh 534005, INDIA. 2Assistant Professor, Department of Biochemistry, NRI Institute of Medical Sciences, Guntur, Andhra Pradesh, INDIA. Email: bhanu.tammineni@gmail.com, drsowji85@gmail.com
Abstract Aim and Objectives: To study the significance of oxidative stress and antioxidants levels in pre eclampsia patients in comparison with normotensive pregnant women. Materials and Methods: Cross sectional study includes 30 pre eclamptic and 30 healthy pregnant women. Fasting venous blood samples were collected during antepartum period and blood levels of Malondialdehyde, Ascorbic Acid, Uric Acid, Superoxide Dismutase And Glutathione Peroxidase is estimated in both controls and cases. Results: In the preeclamptic group, malondialdehyde, a lipid peroxidation product was significantly increased (p<0.001), while antioxidants ascorbic acid and SOD levels were significantly decreased (p<0.001). The other antioxidant uric acid has increased significantly (p<0.001) and Glutathione Peroxidase has no significant change (p>0.05) when compared to normal pregnant women. Conclusion: An altered antioxidant status in Preeclampsia, a indirect proof for the existence of oxidative stress. Key Words: Oxidative stress, Antioxidants, Preeclampsia.
Pregnancy Induced Hypertension (PIH) is a serious complication of the second half of pregnancy that occurs with a frequency of 5-15%.According to WHO, this disease is a leading cause of fetal growth retardation, infant morbidity, mortality and maternal death1 Preeclampsia is defined as a pregnancy-specific syndrome observed after the 20th week of pregnancy with systolic blood pressure of 140 mm of Hg or diastolic blood pressure of 90 mm of Hg accompanied by significant proteinuria (i.e., urinary excretion of 0.3 g protein in a 24-h specimen).In women with Preeclampsia, blood pressure usually returns to baseline within days to weeks after delivery2 It is well known that oxidative stress increases during normal pregnancy. In healthy pregnancy, it has been reported that plasma lipid hydro peroxide levels are increased and total antioxidant capacity is decreased3. More oxidative stress in Preeclampsia results in lipid peroxides, reactive oxygen species and super oxide anion radicals to cause endothelial injury and dysfunction, platelet and neutrophil activation, increased cytokines, superoxide radical production and endothelial damage in a vicious cycle4 Lipid peroxides are generated when free radicals interact with polyunsaturated fatty acids in the cell membrane and in plasma lipoproteins. This process can become self-perpetuating, leading to a cascade of lipid oxidation5. The increased lipid per oxidation leads to the consumption of antioxidants. This leads to reduction in levels of non-enzymatic antioxidants such as Vitamins A, C, E and uric acid as well as enzymatic antioxidants such as glutathione peroxidase and superoxide dismutase5 Uric acid is water soluble and a weak antioxidant. The rise in uric acid in Preeclampsia is not only a non-specific reflection of kidney damage, but also a sign of antioxidative response, possibly related to the pathogenesis of Preeclampsia. The patients with Preeclampsia show hyperuricemia.3 The study was done to determine the changes in serum levels of peroxidation product i.e. Malondialdehyde (MDA) and antioxidant levels namely ascorbic acid, uric acid, superoxide dismutase (SOD) and glutathione peroxidise (GPx) levels in women with Preeclampsia.
MATERIALS AND METHODOLOGY The study was carried out in 30 pre-eclampsia primi patients and 30 normotensive primi pregnant controls who attended the outpatient and inpatient department of Obstetrics and Gynaecology. NRI Institute of Medical Sciences, Guntur, Andhra Pradesh during the year 2012-14. The institutional ethical committee approval was taken. Inclusion Criteria: Cases are preeclampsia primi patients in the age group of 18 to 30 years and with gestation age more than 20 weeks. Controls are normotensive primi pregnant women in the age group of 18 to 30 years and more than 20 weeks of gestation. Exclusion Criteria: Elderly primi gravida subjects, gestational diabetes, chronic hypertension, multiple gestation, those with family history of pre-eclampsia, acute and chronic infections, renal diseases, liver diseases, endocrine disorders, smokers, alcoholics and with history of multivitamin intake. Informed consent was taken from patients and controls. A pre-structured and pre-tested proforma was used to collect the data. Baseline data including age and detailed medical history, clinical examinations and relevant investigations were included as part of the methodology. And appropriate statistical test were applied to study the sample. Blood sample collection: 5 ml plain venous blood sample after overnight fasting was obtained by venepuncture from both cases and controls. This was followed by centrifugation and then sample was processed immediately serum Ascorbic acid and serum Malondialdehyde (MDA) were performed followed by estimation of other parameters by respective methods. Method of estimation of the parameters: Malondialdehyde (MDA)- thiobarbituric acid (TBA) method6 Ascorbic acid- 2, 4 -Dinitrophenyl hydrazine Method7, Superoxide dismutase (SOD)- Pyrogallol at alkaline pH (8.5)8 Glutathione in RBC heamolysate- reduces DTNB to yellow-colored TNB9 Serum uric acid- Brown method10
RESULTS
Table 1: Age wise distribution of cases and controls
The cases and controls are divided into 3 groups (£ 20 years, 21-24yrs, ³25 years). Maximum numbers of cases are in the age group of ³ 25 years (53.34%) and maximum numbers of controls are in the age group of 21-24 years (63.33%).
Table 2: Distribution of gestational age in cases and controls
The cases and controls are divided into 2 groups (22-28 weeks and 29-34 weeks). Maximum numbers of cases are in the gestational age group of 29-34 weeks (56.67%) and maximum numbers of controls are in the gestational age group of 22-28 weeks (53.33%).
Table 3: Blood Pressure Levels in cases and controls
The mean value of systolic blood pressure among cases as compared to controls was statistically significant, (p value < 0.001,t test value 17.02) and mean value of diastolic blood pressure among cases as compared to controls was statistically significant,(p value < 0.001,t test value 28.31).
Table 4: Biochemical parameters to assess lipid peroxidation and antioxidant status of cases and controls
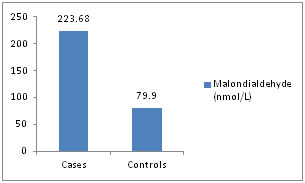
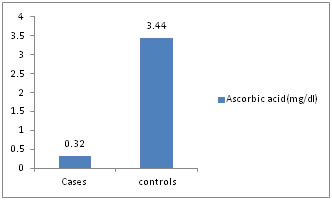
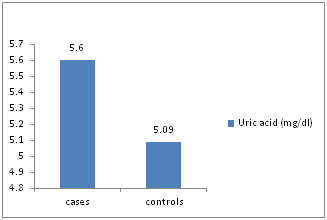
Table 4: The mean serum malondialdehyde (MDA), serum uric acid levels is higher among cases as compared to controls and statistically significant (p value <0.01). The mean serum ascorbic acid, superoxide dismutase levels are lower among cases as compared to controls and statistically significant (p value < 0.01). The mean glutathione levels is higher among cases as compared to controls and not statistically significant (p value >0.05).
Graphical representations Figure 1: Malondialdehyde (nmol/L)
Distribution of cases and controls according to serum malondialdehyde level.
Figure 2: Ascorbic acid (mg/dl)
Distribution of cases and controls according to serum ascorbic acid level.
Figure 3: Uric acid (mg/dl)
Distribution of cases and controls according to serum uric acid level.
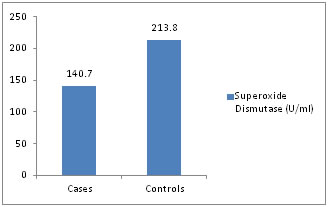
Figure 4: Superoxide Dismutase (SOD) (U/ml)
Distribution of cases and controls according to serum SOD level.
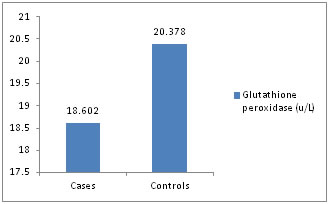
Figure 5: Glutathione peroxidase (u/L)
Distribution of cases and controls according to serum Glutathione peroxidase level. There was a significant increase in plasma levels (P <0.001) of malondialdehyde in preeclamptic pregnancies when compared to normal pregnancies, which was in conformity to study done by Hideaki et al11 and J. T. Uotila et al12,which reported that blood levels of lipid peroxidation products are elevated in women with preeclampsia relative to normal pregnancy. There was a significant decrease in plasma levels (P <0.001) of ascorbic acid in the preeclamptic patients, which was in concordance to study done by Y. Wang et al(13) and Riza Madazli et al14 The study has also shown a significant rise of uric acid (P< 0.001), which was similar to study done by Mehmet Hama et al15 The erythrocyte SOD levels (mean and SD) were 140.7+/-37.70, 213.8+/-68.40 U/ml in PIH, normal pregnancy groups respectively and were found to be decreased n PIH patients compared to normal pregnancy ( p<0.001). The finding are comparable with the various previous studies16,17,18,19 Ilhan et al18 and Kumar and Das17 have shown a decrease in SOD levels in normal pregnancy compared to controls whereas Neena Sharma et. al16 and Hubel et. al19 have shown vice versa. The levels of GPx were 18,602± 6422.74, and 20,378± 5870.8 in PIH, and normal pregnant groups and was not statistically significant (P>0.05), which is in conformity to the various previous studies22,23,24
CONCLUSION In pre-eclamptic patients antioxidants may be utilized to a greater extent to counteract free radical mediated cellular changes, resulting in the reduction of plasma antioxidant levels Measurement of antioxidants status may have important diagnostic and therapeutic implications in PIH. Supplementation of antioxidants during pregnancy in diagnosed PIH cases can minimize the deleterious effects and various maternal and fetal complications of PIH. It can be concluded that measurement of antioxidant status may have possible diagnostic, pathophysiologic, therapeutic implications in PIH.
REFERENCES
|
 Home
Home