Official Journals By StatPerson Publication
|
Table of Content - Volume 5 Issue 3 - March 2018
Apo A1/Msp1 polymorphismsin relation to Apo A-I and HDL-cholesterol levelin coronary artery disease patients with andwithout type II diabetes mellitus
Sachu Philip1*, Philips Abraham2
Professor and HOD, Department of Biochemistry, Vivekanandha Dental College For Women Tiruchengode, Namakkal, Tamil Nadu, INDIA. Professor and HOD, Department of Biochemistry, Al Azhar Medical College, Thodupuzha, Kerala, INDIA. Email: philipsachu1@gmail.com
Abstract A common Mspl polymorphism (G/A) in the promoter region of the APOAl gene (-75 bp) has been shown to be associated with plasma apo A-I and HDL-C variation in several, but not all, studies. Recently another Msp I polymorphic site (± ) in the 5’ region of APOAl ( + 83 bp) has been identified which may also be relevant to HDL metabolism. This study was undertaken to elucidate the individual and combined effects of these two polymorphisms on plasma apo A-I and HDL-C levels in a case control study of 109 coronary artery disease patients. Key Words: Apolipoprotein A-I; coronary artery disease, + 83 bp; - 75 bp
Apolipoproteins are the protein constituent of lipoproteins. Apolipoprotein A-I (apoA-I) is a protein synthesized mainly in the liver and to a lesser extent in the small intestine as a single 243 amino acid polypeptide chain1. It is the major protein found in high density lipoproteins (HDL)and plays an important role in cellular cholesterol homeostasis. It is the obligatory cofactor of the enzyme lecithin-cholesterol acyl transferase (LCAT) and hence a major participant in the regulation of reverse cholesterol transport2. The determination of genetic factors that affect plasma variation of HDL-C and apo A-I levels is critical to further our understanding of the genetic epidemiology of quantitative risk factors for CHD. The important role of apoA-I in reverse cholesterol transport was illustrated by the discovery of the ABC1 gene for ATP binding cassette transporter pathway, a key step in the formation of HDL particles by transporting cellular cholesterol to the plasma membrane followed by incorporation into nascent HDL particles3. ApoA-I also manifests anti-inflammatory and antioxidant effects.4 Epidemiologic studies had shown that both HDL and Apo A-I levels were inversely correlated with the risk of developing CAD.5. Although various factors such as genetic variations, diet, exercise, alcohol, smoking, hormones, and certain drugs significantly influence the levels of HDL and apo A-Ifamily, twin studies of Frank et al have demonstrated a strong genetic heritability, accounts up to 66% of the variability of HDL, and Apo A-I level6. The ApoAI gene is present along with the apoC3 and apoA4 genes, on chromosome 11(11q23.3-qter). DNA sequence variations in the vicinity of APO A l-C3-A gene cluster have been implicated as determinants of plasma HDL-C and apo A-I levels 7. Several polymorphisms in apoA1 have been described and associated with metabolic diseases. One of these common sequence variations resides in the ApoAl gene which involves a G to A transition 75 bp upstream from the start of transcription and creates a site for Msp I restriction endonuclease.8. Recently another Msp I polymorphic site was also identified in the first intron of the apo Al gene9. Two consecutive transitions at + 83 bp (C to T) and + 84 bp (G to A) site occurring together or independently destroy the MspIrestriction site was found to have some influence on apoA I level. It has been shown that the A allele of the apoAI gene contributes to the severity of CAD and low levels of HDL among Northern Indians 10.Hence this study was designed to find out the effect of Apo A1/Msp1 polymorphisms and its relation to Apo A-I and HDL-cholesterol level in CAD patients.
MATERIALS AND METHODS The study was conducted in the department of biochemistry and cardiology of Vinayaka Missions Hospital, Salem. Study group consisted of one hundred and nine individuals with established CAD in the age group of 40-70 years who had undergone coronary angiography and diagnosed with coronary artery disease including single vessel, double vessel and triple vessel and seventy one healthy individuals matched for age, and sex. The subjects were grouped into CAD patients with type 2 DM (CADWDM) ( n=57) and CAD patients without type 2 DM (CADWNDM) (n=52) and normal healthy group(n=71).From each patient, their medical history was obtained through a structured questionnaire and an informed consent was obtained. The ethical clearance was obtained from the institutional ethics committee. Inclusion Criteria
Exclusion Criteria
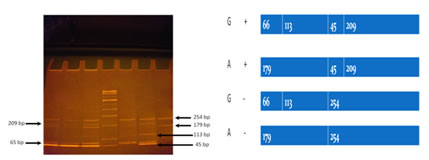
Sample Collection Venous blood sample was collected after an overnight fast of 12 hours and the serum was used for the estimation of fasting blood glucose (FBG) by enzymatic GOD-POD method, cholesterol by enzymatic ‘CHOP-PAP’ method, triglyceride (TG)by enzymatic GPO-POD method and high density lipoprotein cholesterol(HDL-C)by directenzymatic colorimetric method11,12,13,14. LDL-C and VLDL-C were calculated using the Friedewald's formula15.Serum Apo AI was estimated by immunoturbidometric method16. DNA was extracted by adsorption chromatographic method(using commercially available kit).ApoA1 polymorphisms were screened using PCR.A433 base long fragment of ApoAI gene was amplified using a Forward primer: 5’ AGG GAC AGA GCT GAT CCTTGA ACT CTT AAG3’and Reverse primer: 5’ TTA GGG GAC ACC TAG CCC TCA GGA AGA CGA 3’. Subsequently, the PCR amplified fragment was digested with10 U of MspI at 37°C overnight and digested fragments were separated on 10% polyacrylamide gel and was treated with ethidium bromide.DNA was denatured at 95°C for 30 seconds, annealing at 55 ⁰C for 30 sec, and Elongation at 72 ⁰C for 30 sec followed by 35 cycles. The presence of the restriction sites at -75bp (G allele) and +83 bp ( + allele ) resulted in four fragments of 209 bp,45 bp,113 bp and 66 bp. Allele frequencies were identified using Hardy Weinberg equation. All statistical analysis was done using SPSS software,version 16.0. Quantitative variables were demonstrated as Mean± SD. To evaluate the effect of each polymorphism on the variation of quantitative lipid and apoproteinvariables, ANOVA was carried out. RESULTS AND DISCUSSION Table 1 The base line Parameters of Study subjects.
Table 2: Lipid profile andApolipoproteins of study subjects
Table 3: ApoA1 Msp GENE POLYMORPHISM
Allele frequency was measure by Hardy Weinberg equation. Figure 1: Restriction band pattern of SNP ApoA1 gene
Table 4: Frequency of Apo AI gene polymorphism (-75bp)
GG, GA, AA denotes the genotypes. Statistical analysis was done by Chi square test
Table 5: Frequency of Apo AI gene polymorphism (+83bp)
++,-- denotes the genotypes. Statistical analysis was done by Chi square test Table 6: Correlation of Level of Apo AI and HDL-C with Apo AI Gene polymorphism at -75 bp and +83 bp
Statistical analysis was done by Pearson correlation analysis. Correlation is significant at p< 0.05 level.
Among the control subjects 65% were males and 35% were female. Among the CAD WNDM patients 88% were males and 12% were female and in CAD WDM 82% were male and 18% were female. The base line characteristics of study subjects are shown in Table. 1.Age of the study subjects were from 40 to 75 years. The mean age of onset of CAD in the group with type 2 DM was 52 ±9.5 years when compared to 55.4 ± 5.657 years in CAD WNDM. 79% of CAD WDM subjects and 48% of CAD WNDM subjects were of the age group 51-60 years. The mean duration of diabetes in CAD WDM was 6.2±2.5 years. Significant difference in BMI was observed in the CAD WDM subjects when compared to CAD WNDM and control (with p<0.001). HDL-C has been considered as anantiatherogenic lipid factor as it helps in reverse cholesterol transport17 Furthermore HDL particles have been shown to have cardioprotective nature due to its- antioxidant properties, protective effect on endothelial cells, inhibitory effect on endothelial adhesion and activation of leukocytes, inhibitory action on platelet activation 18. In our study, HDL-C level has been found to be significantly lowered in CADpatients when compared to normal. These results draw a parallel with the existing reports 19. Reduced HDL levels have been commonly observed in metabolic syndrome and type 2 diabetes subjects. The reduced HDL cholesterol levels found in CAD WDM may be due to the high Apo E-containing triglyceride-richlipoproteins found inthese patients. ApoE is involved in HDL catabolism and can transfer from triglyceride-rich lipoproteins to HDL. Furthermore, when present on HDLparticles, apoE is predominantly associated with LpAI/AII(Lipoprotein lipase AI/AII) particles. Therefore, the elevation of circulatingapo E-containing triglyceride-rich lipoproteins could lead to increased transfer of apoE to LpAI/AII (Lipoprotein AI/AII) and enhanced catabolism of this fraction.20 Functions and propertiesof HDL particle vary according to its particle size and apoproteins content. It exists as particles of different sizes, with HDL- 2 being the largest and containing the most lipid in its core.The Prospective Epidemiological Study of Myocardial Infarction (PRIME) study examined the association between the incidence of CHD and HDL related parameters, such asapo A-I, HDL A-I, and HDL A-I : A-II 21. All these parameterswere related to CHD risk, however, HDL/apo A-I, and apo A-I werethe strongest predictor. The level of Apo A1 was found to be significantly low ( p<0.001) in CAD WDM compared to CAD WNDM.18% of CAD WDM patients had ApoA1 level >1g/L compared to 38% in CAD WNDM. 82% of CAD WDM subjects had ApoA1 level <1g/L compared to62 % in CAD WNDM. (Table 2). An inverse relationship between the concentration of high-density lipoprotein (HDL) cholesterol and the risk of developing cardiovascular is well established. There are several documented functions of HDLs that may contribute to a protective role of the lipoproteins. These include the ability of HDLs to promote the efflux of cholesterol from macrophages and foam cells in the artery wall and to anti-inflammatory/antioxidant properties of these lipoproteins.22The fact that the main apolipoprotein of HDLs, ApoA-I, plays a prominent role in each of these functions adds support to the view that ApoA-I should be measured as a component of the assessment of cardiovascular risk in humans 23. Moreover there is mounting evidence that HDL subpopulation svaryinterms of their ability to protect against CHD. Case-control studies have suggested that the inverse relationship between HDLcholesterol concentration and CHD is a function of the concentration of the HDL, subfraction24 Level of Apolipoproteins overwhelms thelipids because ApoA1are under more genetic control than lipid components and hence depicts the numberof lipoprotein particles more accurately.25The present study has shown that the level of Apo A1 was significantly low <0.001) in CAD with diabetic subjects when compared to CAD without diabetes. This might be due to the presence of high level of Apo E which cause the catabolism of Apo A1 and HDL. APO-A1 is the major structural protein of HDL(70%)and it has major role is centripetal movement of cholesterol from peripheral tissues including the arterial wall to the liverfor eventual elimination of through the biliary system in to the gut.26The transport of cholesterol and formation of HDL are the basic roles of APO-A1.,low levels of this proteins have been identified as the risk factor in the development and progression of coronary damage.27Apo A1 not only initiates the reverse cholesterol transport by activating the LCAT but also manifests antioxidant and anti inflammatory effects.28It also removes oxidative seeding molecules from endothelium, Scavenges toxic products from arterial wall, ’Reduces smooth muscle cell, apoptosis/necrosis’, Reduces plaque lipid content, Reduces plaque macrophage content and Improves endothelial dysfunction. Furthermore, apo A-I is the ligand for the ATP-binding cassette (ABC) protein, ABCA1,and hence is involved in the docking procedure by which excess cholesterol in peripheral cells is externalized to HDLfor further reverse cholesterol transport either directly or indirectly via LDL back to the liver Hence it can be considered as a better marker than HDL-C 29 Correlation studies of Apoproteins with Lipid parameters in CAD subjects: Correlation analysis between ApoA1 and lipid profile in CAD WDMhad shown a significant positive association with HDL-C (r,.755, p=.000).ApoA1 also showed significant positive association with HDL-C (r,. 415, p=.002) in CAD WNDM. Apo A-1( Apo A1gene,Apo A-1protein0 is the major protein of HDL. The inverse relationship between HDL levels and CAD has been attributed to the role that HDL and its major constituent Apo A-1play in reverse cholesterol transport (RCT).Data has shown that phenotypic expression of ApoA1 depends on the genetic make up. Different polymorphisms in genes coding for proteins related to lipid metabolism may influence the HDL concentration. The G/A polymorphism at the Apo A-I promoter region (-75 bp) is one of the most widely investigated single nucleotide polymorphism (SNP).Another polymorphic site (C/T)was described in the first intron. This +83C/T polymorphism was reported to be associated with apoA1 and HDL levels30 Genotypic distribution of these (-75G/A and +83T/C) polymorphisms were analyzed in our study group. Allele frequencies were identified using Hardy Weinberg equation. In control, CAD WNDM and CAD WDM groups, allelefrequencies for G and A allele were found to be 0.94 and 0.06, 0.81and 0.19 and 0.81 and 0.19 respectively. Frequencies for C and A (+/-) were found to be 0.86 and 0.14, 0.84 and 0.16 and 0.82 and 0.18 respectively. GG++ was found to be predominant genotype in our study group which underlines the fact that it form the wild type in our population. Statistical analysis have shown that no significant correlation exist between the -75G/A or +83C/T polymorphisms of the Apo A1 gene with Apo A1 and HDL level. This polymorphism also lack an association with severity of CAD. Ma et al have reported a positive correlation between C(+) allele and indices of obesity intype 2 diabetes mellitus patients for which reasons were unclear31. In the present study no such association was observed between BMI and gene polymorphism. Pulkkinenet alhad observed a similar result that -75-bp and +83-bp polymorphisms of the Apo A1 gene were not associated with levels of Apo(A1), index of obesityor HDL cholesterol in patients with type 2 diabetes with CHD32. These observations might be due to the lack of influence of this particular gene polymorphism on the phenotypic expression. Moreover, C allele was associated with higher body mass index (BMI) and waist-to-hip ratio in type 2 diabetes subjects. The molecular mechanism by which this intronic polymorphism reduces the indices of obesity is unclear. The -75G/A and +83C/T polymorphisms are in linkage disequilibrium and thus, individuals who carry the rare alleles of the two sites presented higher levels of HDL. The close relationship between altered lipid levels and some elderly diseases, such as obesity and type 2 diabetes, is well known 33 Our findings did not show any association of -75G/A and +83C/T polymorphisms with HDL, LDL, VLDL, total cholesterol and triglycerides levels. Another Brazilian study, which involved a southern population did not find these association either34 In our study we have observed that -75G/A and +83C/T polymorphisms of the Apo A1 gene were not associated with levels of Apo A1 in CAD patients with type 2 diabetes and also in CAD patients without DM.Pulkkinen et al had observed a similar result that -75-bp and +83-bp polymorphisms of the Apo A1 gene were not associated with elevated levels of ApoA1 or HDL cholesterol in patients with type 2 diabetes with CHD32. Studies on ApoA1 gene polymorphism by Kamboh MI et al., andJeenahet al., have found thatpolymorphisms at -75 and +83 bp of the Apo A1 gene have been associated with increased levels of HDL cholesterol and Apo A1 in non diabetic subjects35. Dodani et al has observed that one of the SNP, showed strong association with low HDL that may further increases CAD risk in South Asians36. The association was also found with total cholesterol and LDL, suggesting dyslipidemias as a whole and not just low HDL levels predisposing South Asians to increased risk of CAD. Epidemiologic studies have shown that HDL and Apo A-I levels are inversely correlated with the risk of developing CAD.37 Usis et al have reported that although various factors such as genetic variations, diet, exercise, alcohol, smoking, hormones, and certain drugs can significantly influence the levels of HDL and Apo A-I,family and twin studies have demonstrated a strong genetic heritability, accounting for up to 66% of the variability of HDL, and Apo A-I levels. Our study indicate that haplotype analysis in the 5’region of the ApoA1gene is very useful to uncover the functional significance of this gene in HDL metabolism. Our findings did not show any association of -75 G/A and +83 C/Tpolymorphism with HDL, LDL, VLDL, total cholesterol and triglycerides levels in our south Indian samples. -75 G/A and +83 C/Tpolymorphism did not show any association with ApoA1level. This may indicate that the disturbances in lipid and lipoprotein metabolism in type 2 diabetes could be so profound that the variants in the apo(a1) gene are unable to upregulate HDL cholesterol and apo(a1) levels among these patients. In conclusion, beneficial effects of the -75-bp or +83-bp polymorphisms of the apo(a1) gene are not found in subjects with additional cardiovascular risk factors such as type 2 diabetes. Thus, it is unlikely that the -75-bp or +83-bp polymorphisms of the apo(a1) gene have a major role in determining lipoprotein and apolipoprotein levels or the risk for CHD in patients with type 2 diabetes. Although polymorphisms at -75 and +83 bp of the apo(a1) gene have been associated with increased levels of HDL cholesterol and apo(a1) in non-diabetic subjects, no studies are available on patients with type 2 diabetes. In the present study,the number of subjects with the M22 allele of the apo(a1) gene in the control group was relatively small (n = 3), which could explainwhy the association of the M22 allele and elevated HDL cholesterol and apo(a1) levels was statistically significant only among nondiabetic CHD patients. The 275-bp and183-bp polymorphisms of the apo(a1) gene were not associated with elevated levels of apo(a1) or HDL cholesterol in patients with type 2 diabetes with CHD. In fact, nonsmoking subjects with type 2 diabetes and with the M212/M222 genotype had lower levels of apo(a1) and higher levels of fastingglucose than subjects with the M211 genotype. This may indicate that the disturbances in lipid and lipoprotein metabolism in type 2 diabetes could be so profound that the variants in the apo(a1) gene are unable to upregulate HDL cholesterol and apo(a1) levels among these patients. In conclusion, beneficial effects of the 275-bp or 183-bp polymorphisms of the apo(a1) gene are not found in subjects with additional cardiovascular risk factors such as smoking and type 2 diabetes. Thus, it isunlikely that the 275-bp or 183-bp polymorphisms of the apo(a1) gene have a major role in determining lipoprotein and apolipoprotein levels or the risk for CHD in Finnish patients with type 2 diabetes.
CONCLUSION To stumble and comprehend the genetic epidemiology of quantitative risk factors for CHD. There was marked decrease in the HDL cholesterol level and apo A1 in CAD with DM subjects. This could be due to increased activity of hepatic lipase coupled with hypertriglyceridemia as a result of insulin resistance. Apo A expression in all the study groups did not show marked variations or suggestive genetic polymorphism.
REFERENCES
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home