Official Journals By StatPerson Publication
|
Table of Content - Volume 8 Issue 1 - October 2018
Serodiagnosis of tuberculosis by indigenously developed antibody detection with Indirect ELISA
Kamlesh Manohar Palandurkar1*, Kiran Rajendra Giri2, Reena Rajendra Giri3
1,2Assistant Professor, Department of Biochemistry, IMS BHU Varanasi, Uttar Pradesh, INDIA. 3Associate Professor, Department of Pharmacology, Government Medical College, Akola, Maharashtra, INDIA.
Abstract Background: India is the country with the highest burden of tuberculosis (TB). The World Health Organization (WHO) TB statistics for India for 2016 give an estimated incidence figure of 2.79 million cases of TB for India. We tried to work on rapid diagnosis of TB by developing an indirect ELISA for antibodies against secretory antigens of H37Ra strain which will be indigenous, cost-effective and have better results than market available tests. Materials and Methods: A total of 50 confirmed pulmonary tuberculosis Sputum positive patient were selected. Again 50 sera samples were collected. 50 diseases control sera samples from various diseases (viz., Pneumonia, Lung abscess, Chronic Bronchitis, Non-tuberculous pleural effusion) were taken. We M. tuberculosis H37Ra strain was procured from IMTEK Chandigarh, India and isolated Secretory TB Ag by culturing this bacteria in a protein-free Saton’s medium anti-TB IgG from the sera of TB infected patients. We utilized this TB antigen for the development of Indirect ELISA for tubercular Antibody detection and tested them in above mentioned subject samples. Results: The sensitivity to detect TB Ab in Pulmonary tuberculosis (PTB) cases was found to be 94%. Likewise, the specificity of the test was 88%. The positive predictive value and negative predictive value was 94% and 88.88% and efficacy of the test was 92.20%. Conclusion: We successfully developed and tested indigenously developed Indirect ELISA for tubercular antibody detection. Further work is required to increase stability and effectiveness of this kit and to test its efficacy in extra pulmonary tuberculosis. Key Words: Tuberculosis, Indirect ELISA, HIV, Antibody Detection.
As per the Global TB report 2017 the estimated incidence of TB in India was 28,00,000 accounting for about a quarter of the world’s TB cases. In India in the year 2017 total of 4,35,000 deaths are reported due to Tuberculosis1. This public health problem is the world's largest tuberculosis epidemic2. It is one of the largest on India's health and wellness scale. Disease surveillance in TB is particularly challenging as there is no single reliable method. To be most effective, a multi-pronged approach, combining a number of measures adapted contextually, is required. The government of India has been giving increased emphasis to establishing a strong multi-pronged surveillance system1. Prompt diagnosis followed by effective and complete drug therapy is the only strategy which can stop the TB. Direct sputum smear microscopy by Ziehl-Neelsen acid-fast staining/ Fluorescence Microscopy is the primary case detection tool in RNTCP for patients with infectious tuberculosis presumed to be drug sensitive and is also for monitoring their response to treatment1. The sensitivity of detection has been shown to be only 40-75% and hence clinicians have to treat the suspected patients based on clinical judgment2. Improved diagnostic tests, such as mycobacterial culture and nucleic acid amplification tests are available in developed laboratories which are very few but are often too limited for routine use by TB control programs in India. The Xpert MTB/RIF (Cepheid, Inc., Sunnyvale, CA), recently endorsed by the WHO, is rapid and highly sensitive for the detection of TB and drug resistance; however, this new technology is costly, limiting its use only in the cases where normal treatment of DOTS is unresponsive (MDR and XDR TB)3. In India, there is 14000 smear detection laboratory and only 37 culture and Drug Sensitivity Testing laboratory 28 intermediate references and 06 national reference Laboratories4. All these are surely outnumbered for testing large population affected by tuberculosis in India. Therefore, rapid and reliable diagnostic tests for the detection of tuberculosis is the need of time. The diagnosis of TB by serological methods can be a great alternative to sputum testing. Serological tests are relatively simple to use and easy for interpretation. They have been used in several diseases like malaria and HIV5. They are easy to use and store and need a little expertise. So they present a very good option for field testing and surveillance. The WHO Expert Group meeting report July 2010 strongly encouraged further research to identify new/alternative and accurate point-of-care serological tests with improved accuracy6. Antibody detection tests have the advantage that it provides direct evidence of active disease based ion titer, thus allowing for immediate initiation of TB treatment. Antibody detection is done commonly by indirect enzyme-linked immunosorbent assay (ELISA) technique, also called antibody-capture ELISA7. The humoral response to Mycobacterium TB presents an antibody in human serum for after the tuberculosis infection. This antibody is more specific for tubercular Ag than the antibody raised in animal models. Antibody detection serological assays that use single tubercular antigen that may not recognize all tubercular infection. So the development of indirect ELISA based on the use of cocktails secretory antigen may increase the sensitivity and specificity. With this hypothesis, we tried to develop ELISA to detect tubercular antibody in serum of tuberculosis.
MATERIALS AND METHODS Subjects/Materials: Institutional Ethical Committee approved the study after review and presentation and the work for this project started in Oct 2008and finished in September 2010. 50 Pulmonary tuberculosis (PTB) patientssputum positive including HIV-TB co-infection cases (n=7), 50 disease control patients (viz., Pneumonia, Lung abscess, Chronic Bronchitis, Non-tuberculous pleural effusion) after excluding Tuberculosis, 30 healthy control subjects were included in the study from the Department of Tuberculosis and Chest Medicine, Acharya VinobhaBhave Rural Hospital, Sawangi (Meghe), Wardha after obtaining written consent for enrollment in the study. Healthy control subjects were apparently healthy at the time of sample collection and not suffering from any diseases for the last one year. Clinical history (includes present, past and contact history), Treatment History (Anti tubercular treatment [ATT] under DOTS) of all the subjects (case, disease control and healthy control) was obtained. Blood investigations (HIV-ELISA), sputum sample tests(includes AFB smear test), Radiological investigations (chest X-ray, CT-Scan, USG- Abdomen), were also done for confirmation of their designated group of study. Methods: Mycobacterial strain used: The M. tuberculosis H37Ra strain was procured from IMTEK Chandigarh, India. Culture: The bacilli were grown on the L-J medium for a period of 3-4 weeks 370 C in bacteriological incubator there was ample growth on the slants and Rough buff tough colonies peculiar of the Tuberculosis bacilli were visible (Fig-I). Further sub culturing the bacilli purity of the culture was verified by bacteriological examination viz., ZN staining (Fig-III) and Gram staining. Turbidity of the media increases gradually so as to give an opaque appearance of the media at the end of 7th day of sub culture. Isolation of M. tuberculosis Secretory Antigen: The bacilli were separated from the medium by filtrating with Whatman 3 filter paper. Finally the culture was totally freed of bacilli by sterile membrane (pore size 0.22µ) filtration. Culture filtrate was dialyzed extensively against 0.01M PBS, pH 7.2 for 24 hrs at 40 C. Molecular weight cut off of 10-12 kDa. The dialyzed culture filtrate was concentrated 25 fold by ultrafiltration using membrane of 10-12 kDa molecular weight cut off (Sigma). The protein concentration was estimated by Bradford Method and labeled as M.tuberculosis Secretory Ag and stored in aliquots at -200 C with sodium azide (1mg/ml) and proteolytic inhibitor cocktail (10µl/ml) (Sigma). Collection of blood samples, processing and its storage: 2 ml of venous blood was collected from each individual after taking informed consent in a plain bulb with all the aseptic precautions and kept for settling to coagulate for at least 15-20 minutes. The sera was separated from this by centrifuging the coagulated blood at 3000 rpm for 10 minutes and stored separately in an aliquot after adding 10% sodium azide (NaN3) (S D fine chem. Pvt Ltd, Mumbai) 10μl/ml and 10μl/ml protease inhibitor cocktail (Sigma) as preservative. Precautions were taken for avoiding the contamination of the sample and for avoiding the spread of tuberculosis infection. Each sample is linked by a unique numerical code for detailed clinical information. Indirect ELISA: The enzyme linked immunosorbent assay (ELISA), first developed by Engvall and Perlman 8 (1971) is a heterogeneous enzyme immunoassay that uses solid phases e.g. plastic tubes, beads, microplates, membranes such as cellulose acetate membranes. The test sample (serum or other body fluids) is thenincubated and antibody bound to immobilised antigen is detected with enzyme labelled second antibody. Then result specific substrate is added to develop colour and colour intensity is being measured. Indirect ELISA quantitation of tubercular antibody (IgG): Protocol
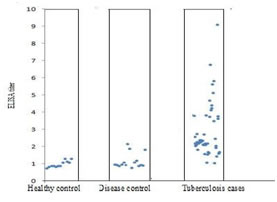
RESULTS The individual ELISA titre is shown by Scatter gram (Fig-1). Each dot of the Scatter gram represents individual subject’s ELISA titre reading. X-axis represents the number of subjects of that particular group and Y-axis represents the corresponding ELISA titre readings. We did indirect ELISA with M. Tuberculosis H37Ra secretory antigens for the detection of tuberculosis as described in materials and methods. Following are the results with this test. We have taken the ELISA titer ratio as a comparative tool so as to avoid the intra assay variations. Pooled healthy sera was always taken as a standard in each batch as a comparison. At first we run 30 healthy control samples and then took their ELISA titer ratio. Their mean and SD were calculated and mean + 2S.D. was taken as cutoff for the differentiation of positive and negative result. Mean of 12 healthy control samples ELISA titer ratio is 0.9625. The Standard Deviation of 12 healthy control samples is 0.184. The Cut-off (Mean +2 S.D.) thus calculated is 1.33. The mean and standard deviation of the ELISA titres of PTB cases, DC cases and HC subjects were calculated. The mean ELISA titre for PTB cases was 2.25 and SD was 0.93. The lowest ELISA titre in PTB cases was 0.75 while the highest one was 4.06. The mean ELISA titre for DC cases was 1.24 and SD was 0.62. The lowest ELISA titre in DC cases was 0.59 while the highest one was 3.01. (Fig:2) In 47 out of 50 PTB cases, TB Ab can be detected accurately by our indirect ELISA but 09 out of 50 DC cases gave false positive result. None of the HC group subjects had their ELISA titre above cut-off (>1.06) which shows the high specificity of the test in healthy individuals. The Sensitivity to detect TB Ab in PTB cases was 94% and the Specificity for PTB was same 88%.The positive predictive value and negative predictive value was 94% and 88% respectively. These values of both the groups is represented in cylindrical column-diagram (Fig:3). The ROC curve gives an idea of the overall performance of a test across different thresholds. The closer the curve is to the upper left hand corner of the plot (sensitivity and specificity both 100%), the better the performance of the test. The area under the curve for our test is 92% which means that our developed test is effective. (Fig:4) HIV test results were also available from 50 out of 50 case patients, of which 06 were positive (14%), and from 50 out of the 50 control subjects with non-tuberculous pulmonary disease, of which none were positive. Our test detect the TB Ab in 95% (35) of the HIV uninfected patients of PTB cases group while only 66% [n=4] of the HIV-TB co-infected patients of PTB cases group. The Indirect ELISA test result in HIV uninfected PTB cases and in HIV-TB co-infected cases is shown by cylindrical column-diagram (Fig: 5). Scatterogram of the Comparative ELISA titers of the Subjects in various study groups
Figure 1: Scatter graphic representation of the ELISA titre of all the study groups (ELISA titre: <1.06=negative result and> 1.06=positive result)
Figure 2: The mean ELISA titer for the Healthy controls was 0.815. The mean ELISA titer for the disease control was 0.88. The mean ELISA titer for the Tuberculosis cases was 2.02.
Figure 3: The Receiver Operating Characteristic (ROC) curve, which represents the variation of the sensitivity and specificity according to the cut-off value, was established for the group of PTB. (Area=0.82) DISCUSSION India bears one-quarter of world’s TB cases which is an alarming situation. Also we came to know that the numbers of TB cases are on rise in our rural based tertiary care hospital. The use of serologic methods to diagnose tuberculosis have been studied since 1898 (9) which was first introduced by Arloing as a technique of hemagglutination. Owing to the basic nature of the disease and the diversity of the host immune response, antibody detection can be of practical use12. To develop serological methods for the diagnosis of TB, different antibodies have been evaluated. Following are some of the antibodies those have been tested by various researchers in their studies:-rabbit anti-purified protein derivative-RT 23 IgG and enzyme penicillinase, polyclonal anti-BCG rabbit Ab and HRPO conjugated monoclonal Ab, affinity purified anti-H37Rv Ab raised in-house in rabbits using cell wall material of MTB, murine monoclonal Ab against LAM, affinity purified antibodies against Mycobacterium tuberculosis H37Ra antigens (SEVA TB ES-31, ES-43 and EST-6); - for the detection of TB Ag in sera of the patients (11, 12, 13, 14,15). We detected the tubercular specific antibody from the sera of tubercular infected patients for the capture of tubercular secretory antigen of H37 Ra Strain for our indirect ELISA.The initial cocktail antigen could be used to capture the antigen, followed by the detection of anti-mycobacterial antibody by the second peroxidase-labeled antibody. Also, preparation of such antibodies for use in indirect ELISA for screening could be done without sophisticated technology as described. Although various immunodiagnostic techniques, based on the detection of mycobacterial specific antibodies have been reported, they have not come into wide spread clinical use. The reagents used in some of them are not generally available and were difficult to prepare. With the use of generally available reagents, the test would be suitable as a routine diagnostic test of tuberculosis18. In our study, we had developed the indirect ELISA with the use of commercially available routine reagents. We evaluated our developed Indirect ELISA for the antitubercular Ab detection from the serum of tubercular patients. The sensitivity of our Indirect ELISA is well within higher range of the sensitivities of other worker’s antigen detection ELISA i.e, from 37%-95% and the specificity is well within the other’s work specificities i.e., from 73%-100% respectively13-20. The accuracy of the predictive value of our ELISA, however, was indicated by the fact that readings for only 03 of 50 sera specimens were incorrect. Specificity was very high in healthy individuals and was not compromised by tuberculin sensitivity or childhood BCG vaccination. Global tuberculosis morbidity and mortality remain high and in many parts of the world are increasing because of co-infection with human immunodeficiency virus17. Our Indirect ELISA has achieved the goal of WHO recommendations in relation to sensitivity of serological test for the replacement of culture to diagnose PTB cases without HIV infection21. In HIV-uninfected patients, combined use of serology and sputum microscopy was as sensitive as culture, thus representing an opportunity to greatly shorten the time for diagnosis in a substantial subset of patients. It is interesting that patients with HIV have less antigenemia than patients without HIV, since the load of mycobacteria is probably greater in HIV positive patients. Because of their immunosuppression, patients with HIV are not able to degrade mycobacteria and therefore do not release antigen in amounts necessary for detection by the test which might be a reason of low sensitivity in HIV-TB co-infection cases.20 The serology test result was positive in 06 of the 50 sera from disease control patients, all of whom were from the subset with symptomatic non-tubercular pulmonary disease. In addition, false positive reactions could occur in the assay employed in the study, as the first and second antibodies used in our Indirect ELISA were obtained from the same species18. In antibody detection assays, sample processing is an important, but often laborious and time-consuming, step. Mycobacterial antigens have been found as components of circulating immune complexes, so it may be necessary to dissociate the immune complexes to achieve a higher sensitivity in the immunoassay20. There are some limitations to the current study. But the fact remains unquestioned that the presence of antigen is the only hallmark of presence of active disease. Hence, immuno-detection of circulating M. tuberculosis antigen secreted during active infection could be better diagnostic marker than antibody. Sensitivity has improved in our study as compared to other antibody assay 275 but specificity has reduced as compared to the antibody assay against single antigen. Thus we can conclude that in the quest of increased sensitivity we have lost specificity for the disease. But as this was the small sample we will continue our study for the better evaluation of our method.
CONCLUSION In summary, we have shown that anti IgG for secretory proteins is a sensitive marker of active TB considered to be at increased risk for tuberculous infection. Anti-tubercular secretory proteins IgG was quite specific for active disease. The high negative predictive value of anti-tubercular secretory proteins IgG in a population at risk for tuberculous infection makes it a potentially valuable screening test for active TB. In the present study using cocktail of antigens for their antibodies, has shown good diagnostic potential and thus useful in confirming Tuberculosis along with other clinical and laboratory findings. Screening of sera for anti-tuberculosis IgG antibody to cocktail antigen was found to be useful in detection of tuberculosis.
REFERENCES
|
 Home
Home