|
Table of Content - Volume 4 Issue 1- October 2016
Triglycerides levels in schizophrenia: A comparative study among untreated, treated and their first degree relatives
B Ramakrishna1, Murali Krishna V2, Vijay Kumar M3, Raghuram Macharapu4*
{1Assistant Professor, Department of General Medicine} {2,3Post Graduate, 4Associate Professor, Department of Psychiatry} Mamata Medical College, Khammam, Telangana, INDIA. Email: raghuram.macharapu@gmail.com
Abstract Objective: To compare any differences in the lipid profile of untreated schizophrenia patients, treated schizophrenia patients and their first degree relatives. Methodology: The Study is conducted among untreated schizophrenia patients, treated schizophrenia patients and their first degree relatives who came to Mamata general hospital, Khammam. Total study sample consists of 90 out of which there are 30 treated schizophrenic patients, 30 untreated schizophrenic patients and 30 first degree relatives of either group who were randomly selected. Estimation of Triglycerides by Enzymatic Colorimetric Test: (GPO/PAP method). Results: Mean triglycerides was significantly higher in treated schizophrenics compared to untreated schizophrenics and FD. There was significantly higher rate of hypertriglyceridemia in patients treated with anti-psychotics when compared to drug naive and first degree relatives. There were no significant differences in rate of hypertriglyceridemia between FDR and drug naive patients. Conclusion: Use of second generation anti-psychotics in schizophrenia patients significantly affects lipid profile, which increase triglyceride levels abnormally. Key Words: Anti-psychotics, schizophrenia, lipid profile, triglycerides.
INTRODUCTION Schizophrenia is a clinical syndrome of variable, but profoundly disruptive, psychopathology that involves cognition, emotion, perception, and other aspects of behavior. The expression of these manifestations varies across patients and over time. But the effect of the illness is always severe and is usually long lasting. It probably causes more suffering and distress and blights more lives than any other cancer and certainly represents a major burden for care-givers, health services and society as a whole.1 Antipsychotic medications are the mainstay of treatment for psychotic illnesses and are also widely used in many other psychiatric conditions. Introduced about 50 years ago, these medications have helped millions of people manage their symptoms. For people who respond well, antipsychotics can mean the difference between leading an engaged, fulfilling community life and being severely disabled.2 The first-generation antipsychotics (FGAs) are still widely available and are effective at treating positive symptoms of psychosis, such as hallucinations and delusions. FGAs do not however, adequately alleviate many other common and important aspects of psychotic illness, such as negative symptoms (e.g., withdrawal, apathy, poverty of speech), cognitive impairment and affective symptoms. In addition, all FGAs can produce significant extra pyramidal side effects at clinically effective doses. These side effects, which include dystonic reactions, drug-induced Parkinsonism, akathisia and tardive dyskinesia, can make treatment intolerable for some people, leading to subjective distress, diminished function, stigma and nonadherence.2 The introduction of second-generation antipsychotics (SGAs) or “atypical “antipsychotic drugs promised enhanced efficacy and safety. The newer agents appear more efficacious than conventional drugs in reducing negative symptoms (e.g. lack of emotion, interest, and expression). The safety advantages of the atypical drugs have been questioned because of their propensity to induce weight gain and alter glucose and lipid metabolism.3 Abnormal lipid biology may play a significant role in the pathophysiology of schizophrenia. Most studies show that patients with schizophrenia have higher levels of serum lipids (cholesterol and triglyceride) than a healthy population.4,5 This dyslipidemia has been regarded as a result of antipsychotic medication and lifestyle factors6, but dyslipidemia has also been demonstrated in unmedicated schizophrenia patients.7,8,9,10 Altered metabolism of membrane lipids (polyunsaturated fatty acids, PUFA) is another aspect of lipid biology suggested to be involved in schizophrenia pathophysiology.11 Lower levels of PUFA in cell membranes have been found in schizophrenia12,13, both in the acute and chronic stage of the disease.14 The course and outcome of schizophrenia is regarded as heterogeneous. The nature of the relation between lipid profiles, lipid metabolism and clinical characteristics of schizophrenia is mainly unknown. Particularly, it is not known whether lipid profiles are associated with the disease itself, and / or current symptoms. Conflicting findings of associations between lipid levels and symptom severity may represent fluctuations of lipid levels as the disease progresses.15,16 The aims of the current study are to determine if there are differences in lipid profiles of untreated schizophrenia patients, treated schizophrenia patients and their first degree relatives.
MATERIALS AND METHODS Place of study: Mamata general hospital, Khammam. Study period: The study period is from January 2014 to December 2014. Study sample: A total of 90 study sample were included in the study. Study design: Cross sectional study. Inclusion Criteria
Exclusion Criteria
Materials: A semi-structured demographic Proforma with age, gender, marriage status used. Estimation of Triglycerides: Enzymatic Colorimetric Test: (GPO/PAP method). The study was approved by the research ethics committee. Subjects were briefed in detail about the nature and purpose of the study. Confidentiality was assured and informed consent was taken. Statistical Analysis: The data was analyzed using SPSS software version 17.0. Descriptive results are expressed as mean and SD of various parameters in different groups. Multiple comparisons ANOVA was used to assess the significance of difference of mean values of different parameters in between groups. F value was used to calculate the significance in between groups. Significance < 0.05 was considered as significant and level > 0.05 was considered as non significant. Chi-square test was done for comparison of distribution between the groups. Significance < 0.05 was considered as significant and level > 0.05 was considered as non significant.
RESULTS
Table 1: Distribution of sample based on age
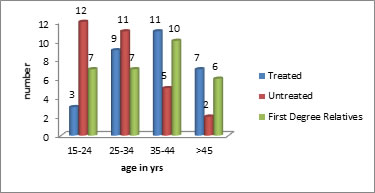
Figure 1: Distribution of sample based on age
Table no-1 and Figure-1 depicts that majority of patients in treated group were in the age group of 35-44 yrs. majority of patients in the untreated group were in the age group of 15- 24yrs and majority of subjects in FDR group were in the age group of 35-44 yrs. There was no significant difference in the mean ages between treated, untreated and FDR group (p>0.05).there was no significant difference in the distribution of sample between the three groups based on age groups.
Table 2: Distribution of sample based on gender
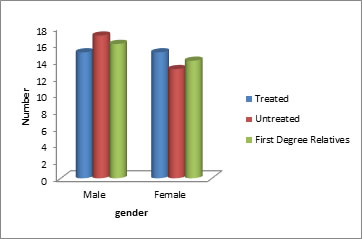
Figure 2: Distribution of sample based on gender
Table no-2 and Figure no-2 depicts that treated group comprised 50 % males and 50% females, untreated group comprised 56.7 % males and 43.3 % females and FDR group comprised of 53.3% males and 46.7 % females. There was no significant difference in gender distribution in either of the groups (chi square = 0.268, p=0.87).
Table 3: Distribution of Triglycerides levels in the sample
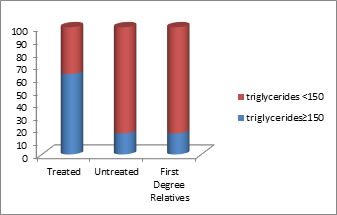
Figure 3:
Table 4: Mean±SD Values of Triglycerides in controls and cases
Table 5: ANOVA multiple comparison of significance for triglycerides
DISCUSSION In present study we observed Mean triglycerides was significantly higher in treated schizophrenics compared to untreated schizophrenics and FDR. There was no significant difference in mean triglycerides between untreated schizophrenics and FDR as depicted in table no-6. This explains that dyslipidemia in the form of hypertriglyceridemia occurs more frequently in patients on therapy with the new generation of antipsychotics. Table no 3 and figure no 3 depicts the distribution of hypertriglyceridemia in the sample, in the present study it was observed that 63.3% of AAP treated schizophrenic patients had (tg≥150) when compared to 16.6% in drug naive schizophrenic patients and 16.6% in first degree relatives who were taken as controls. There was significantly higher rate of hypertriglyceridemia in patients treated with anti-psychotics when compared to drug naive and first degree relatives. There were no significant differences in rate of hypertriglyceridemia between FDR and drug naive patients. The present study has shown that dyslipidemia was represented in the form of hypertriglyceridemia in patients on therapy with the new generation of antipsychotic drugs compared with patients treated with antipsychotics. Despite the adverse effects or the impact of new antipsychotics, primarily clozapine and olanzapine in the development of hyperlipidemia and obesity, a number of factors should be considered when choosing among the antipsychotic medications. (17) These include the nature of the patient’s psychiatric condition, specific target signs and symptoms, past history of drug response, patient preference, history of treatment adherence, medication effectiveness, psychiatric and medical comorbidities, availability of appropriate formulations, need for special monitoring, and cost of and access to medications.18,19 The population of schizophrenic patients is at greater risk for developing obesity, type-2 diabetes, dyslipidemia and hypertension in the general population20 The relationship between weight gain caused by antipsychotic drugs and the occurrence of dyslipidemia is not yet entirely clear. Some studies suggest that weight gain and metabolic syndrome are certainly associated with the treatment with antipsychotics, but the occurrence of dyslipidemia was significantly associated with antipsychotic treatment.21 In several studies it was found that in patients who were not obese and were not treated with antipsychotics, the concentration of triglycerides for two weeks after the treatment with the new generation of antipsychotics significantly increased.22,23 Most antipsychotics may cause dyslipidemia indirectly through an increase in body weight, leading to abdominal obesity and hyperlipidemia.24 One study which compared 38 drug naive schizophrenia patients with 44 matched healthy controls found no differences in HDL, or triglycerides.25 Sengupta et al26 in a Study on first episode psychosis (FEP) patients also did not find any lipid derangements except a slight trend of lower HDL levels. A study on chronic patients of schizophrenia in community, who were never treated, also failed to find any significant lipid disturbances in patients compared to healthy controls.27 One study reported abnormal lipid profile in patients undergoing antipsychotic treatment.28 One study found increased triglycerides in atypical antipsychotic treated patients when compared to untreated even after adjusting for risk factors for hyperlipidemia.29 Sheitman et al30 did not observe any change in lipoprotein levels but the level of triglyceride increased from a mean of 170 mg/dl to 240 mg/dl after initiation of treatment with aAAP (olanzapine). Similar to the findings of present study, one study found that patients treated with atypical antipsychotics had a significant Prevalence of hypertriglyceredemia compared to controls. The treated group had 4 times higher odds of being diagnosed with hypertriglyceridemia.31 Rates of triglyceridemia in this study are higher than that of Gupta and co-workers(32) which found increased prevalence of hypertriglyceridemia (44%) in patients treated pith antipsychotics. One meta-analysis reported on hypertriglyceridemia. The Prevalence of hypertriglyceridemia was 34.5% in schizophrenia. Medicated patients had significantly higher rate of hypertriglyceridemia when compared to drug naive patients and general population.33 Findings of lipid derangements in this study are higher ‘than a meta-analysis which reported, the rate of hypertriglyceridemia in undedicated patients was 16.9% and the proportion of those with low HDL was 20.4% compared to medicated patients in whom it was 41.1% and 44.7%.33,34
CONCLUSION In present study we observed Mean triglycerides was significantly higher in treated schizophrenics compared to untreated schizophrenics and FDR. There was significantly higher rate of hypertriglyceridemia in patients treated with anti-psychotics when compared to drug naive and first degree relatives. Use of second generation anti-psychotics in schizophrenia patients significantly affects lipid profile, which increase triglyceride levels abnormally.
LIMITATIONS Study sample was collected from only one tertiary care hospital, which was the major limitation of the study and further research can be conducted, so results cannot be generalised to the population.
REFERENCES
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home