|
Table of Content Volume 13 Issue 2 - February 2020
Characterisation of streptozotocin induced diabetes mellitus in Wistar albino rats - A histological and haematological perspective
Rajesh R1*, Sreekala V2
1Assistant Professor, Department of Anatomy, KVG Medical College, Sullia, Karnataka, INDIA. 2PhD Scholar, Department of Dravyaguna, IMS, BHU, Varanasi. Uttar Pradesh, INDIA. Email: rajesh2361@gmail.com
Abstract The present study was designed to investigate the characteristics of Diabetes Mellitus by Streptozotocin induced diabetic rats as assessed by blood glucose concentration and structural changes in the pancreatic islets. Wistar albino rats of either sex were intraperitoneally administered single dose of Streptozotocin 130 mg/kg (Group I), 100 mg/kg (Group II) and 65 mg/kg (Group III) respectively. Persistent hyperglycemia with above the range of 180 mg/dl was observed during the entire experimental study period in all the groups. Severe to moderate levels of mortality rates were observed among Group I and II by 6 weeks. Whereas no causality was observed in animals in Group III. The histopathological studies of pancreas showed severe structural damages in the is lets of group I and II. Whereas moderate beta cell damage was observed in Group III. Therefore, it is 65 mg/kg b.w injection is appeared to be safe and effective dose to create a diabetic at model for long term diabetes studies. Key Word: Diabetic rat model, Hyperglycemia, Pancreatic islets, Streptozotocin.
INTRODUCTION Diabetes Mellitus is defined as ‘a metabolic disorder characterized by hyperglycemia resulting from defects in insulin production, insulin action, or both1’. It is also associated with hyperlipidemia and hyperaminoacidemia2. Diabetes Mellitus causes derangement of protein, lipid and carbohydrate metabolism, that eventually leads to many secondary complications such as Dyslipidemia, Coronary Artery Disease, Renal Failure, Stroke, Neuropathy, Retinopathy and Blindness.The typical symptoms of hyperglycemia in Diabetes mellitus are polyuria, polydipsia, polyphagia and weight loss3,4. Streptozotocin is widely used for the development of both NIDDM and IDDM in animal models5, and produces different degrees of Diabetes depending on dose administered either by i.v or i.p injections6,7. The severity of the diabetes depends on the dose of streptozotocin injection8, but many instances extreme hyperglycemia for longer period may produce mortality and morbidity of the rats. The search of a effective animal diabetic models with persistant hyperglycemia and pancreatiuc islet damage without any mortalities is a hot subject in the therapeutic preclinical animal studies. The present study is an attempt to explore the characteristics of Diabetes mellitus induced in Wistar rats by a single intraperitoneal injection of streptozotocin in three different doses to design a safe and effective animal model.
MATERIALS AND METHODS Laboratory bred neonatal albino rats of Wistar strain (Rattus Norvargicus) of both sexes were obtained from the Central animal house, Mahatma Gandhi Medical College and Research Institute. The pups were housed, well maintained, and kept with their respective mothers in polypropylene cages under standard laboratory conditions. This study was approved by the Institutional Animal’s Ethics Committee of Mahatma Gandhi Medical College and Research Institute, SBV University, Puducherry, and CPSEA. Induction of Diabetes Mellitus Streptozotocin (STZ) 100 mg (Sigma, Aldrich – USA), which is in the powdered form, kept in a deep freezer (-200c) to prevent the degradation. After weighing out enough STZ for all animals to be injected on the day in a microfuge tube, the vials were wrapped carefully with a silver foil to prevent degradation by light exposure. On the day of injection, the STZ was dissolved in ice cold 0.01 M citrate buffer, pH 4.5 and prepared freshly for immediate use within 5 min. STZ injections were given intraperitoneally and the doses were determined according to the body weight of the rats.The injection was given in the lower right quadrant of the abdomen to avoid damage to the urinary bladder, cecum and other abdominal organs(9). Animals were kept in poly propylene cages under standard laboratory conditions with pelleted diet and water ad libitum. The blood samples were collected from the tail vein once a week using a one – touch gluco meter (Accu-Chek, Roche diagnostics). and the animals showing BGL more than 180mg /dl on the second post injection day were considered as diabetic(10). Experimental Groups and Protocol The animals were dispersed in to four experimental groups. Each group comprised of 10 rats in the beginning of the study. Animals in group I were intraperitoneally administered single injection of 130 mg/kg of STZ. Animals of group II were intraperitoneally administered a single injection of 100 mg/kg of STZ. Animals of group III were intraperitoneally administered 65 mg/kg. Animals of group IV served as control group were injected with equivalent amount of cold citrate buffer (pH 4.5). All the doses of STZ were administered at a volume not exceeding 10ml/100 g body weight of rats. After 6 weeks of blood glucose measurements animals were sacrificed by painless cervical dislocation under mild ether anaesthesia. Pancreas was carefully dissected out and fixed in 10% neutral buffered formalin and processed for routine histological studies.
STATISTICAL ANALYSIS The data were expressed as Mean ± SEM (Standard Error of Mean). Statistical differences between groups were analyzed using one-way Analysis of Variance (ANOVA) followed by Tukey’s HSD, for pair wise comparison. The variance was considered statistically significant at p value < 0.05. To obtain comparable results, data of six rats from each group was used for statistical analysis.
OBSERVATIONS AND RESULTS The values of blood glucose concentrations are shown in Table 1. All the animals were weighed weekly and their general conditions were also monitored throughout the experimentals. Table 1: Blood glucose concentrations in experimental rats
The data were expressed as Mean ± SEM (Standard Error of Mean) of six experiments. P < 0.05 when compared to control. Effect of STZ Injection (130 Mg/kg ) on Blood Glucose All the animals developed diabetes mellitus 2 days after administration of 130 mg/kg STZ. A significant rise in blood glucose concentration was observed from the first week onwards and it was progressive, but the severity of diabetic status was highly fluctuating within the group. By the completion of the study period 40% mortality was observed. The percentage of causalities were relatively more at the final 2 weeks. The results included only the data of six rats survived till the end of the study. Effect of STZ Injection (100 Mg/kg) on Blood Glucose All the animals developed diabetes mellitus 2 days after administration of 100 mg/kg STZ. A significant rise in blood glucose concentration was observed from the first week onwards and it was progressive with moderate to severe fluctuations.by the completion of 6th week 20% mortality was observed. The results included only 6 of those animals survived till the end of the study. Effect of STZ injection (65 mg/kg) on blood glucose: All the animals developed Diabetes Mellitus two days after the administration of 65 mg/kg of STZ. A steady but significant rise of blood glucose levels with moderate fluctuation within the group. No casualties were observed among the group and the general outlook of the animals were appeared near normal. Effect of STZ injection on the microstructure of Pancreas The pancreas of the untreated rats showednormal architecture with large Islets of Langerhans with cords of beta cells with blood vessels (Fig-1 A).Whereas the pancreatic islets of the animals treated with 130 mg/kg b.w STZ appeared very small irregular and degenerated vacuolations and necrosis were evident in the centre with few infiltratory cells (Fig-1 B) and the rats treated with 100mg/kg STZ also showed severe degeneration of pancreatic islets leaving few cells in the centre with less vacuolations(Fig- 1 C), but many of the nuclei showed pyknosis; and with 65 mg/kg b.w dose, animals showed moderate degeneration of the islets with minimum vacuolations or necrosis with better hisotarchitecture (Fig-1 D). 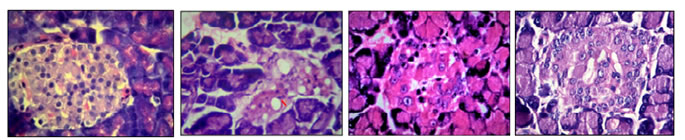 A B C D
Figure 1: The effect of STZ Pancreatic islets. a) Normal pancreas showing rounded islets with centrally placed cords of beta cells B) STZ 130 mg treated rats showing severe degenerated crumpled islets with necrosis (N) and vacuolations(V), C) STZ 100mg treated rats showing severe degeneration of islet with pykntic nuclei. D) 65 mg /kg treated rats showing rounded islets with moderate damage. Streptozotocin (STZ) ( 2- Deoxy – 2 - ( 3 – methyl – 3 - nitrosourea ) 1- D – glucopyranose), a cytotoxic glucose analogue, derived from Streptomyces Achromogenes11. Generally, it appears as off-white or light yellow crystalline powder. Before it was being used in the treatment of malignant pancreatic islet cell tumours and other malignancies. Now it has been widely used as a diabetogenic agent to produce experimental diabetes in laboratory animals such as guinea pigs, mice, monkeys, rabbits, hamsters and rats12. The mechanism of action of STZ that results in specific beta cell toxicity is attributed to the glucose moiety in its chemical structure that allows it to enter the beta cells through the low affinity GLUT 2 (glucose transporter 2) receptors in the plasma membrane13. There are three main pathways by which streptozotocin targets the cells. These are (a) NAD+ depletion by forming carbonium ion as a result of DNA methylation (b) Streptozotocin will act as nitric oxide donor in beta cells leading to damage and DNA alkylation by producing Nitric oxide (c) production of Free radicals or Reactive Oxygen Species (ROS)14 i.e., ROS are produced during glucose auto-oxidation and protein glycation, which in turn results in tissue damage15,16. STZ treatment also increases in malonaldehyde (MDA) activities that intensifies the vulnerability of pancreas to oxidative stress17. a single high dose of streptozotocin is capable of producing rapid irreversible necrosis of insulin producing beta cells of the islets of Langerhans, thus eventually generatingInsulin Dependent Diabetes Mellitus. There are many reports on the mortalities associated with the high dose streptozotocin injection due to the development of ketoacidosis in the experimental animals18. On the other hand, low dose of streptozotocin < 50 mg /kg b.w in adult rats is ineffective to produce persistent hyperglycemia for a longer duration14,19. In the present study, we have seen the diabetogenic response in rats with three different models used in experimental diabetic studies, i.e. 130, 100 and 65 mg/kg respectively to determine a suitable model that can produce persistent hyperglycemia without or least mortality, for long term diabetic experimental animal studies. The increased numberof casualties among the rats injected with 130 mg /kg b.w may be attributed to the ketoacidosis due to hyperglycemia which is been reported by many authors20,21. The severe damage seen in the pancreatic islet is due to the specific beta cell toxicity of the STZ. The degree of beta cell toxicity is directly proportional to the dose of STZ single intraperitoneal injection of 65mg kg b.wof STZ is able to produce persistent hyperglycemia with moderate amount of pancreatic islets damage has been reported by different authors22.The present study supports their findings this is further reinforced by the moderate structural changes in the beta cells of the central part of the islets that is occupied by the insulin producing beta cells;
CONCLUSION Intraperitoneal injection of STZ at 65 mg /kg b.w was appeared to be safe and effective when compared to higher doses such as 130mg/kg and 100 mg/kg. As streptozotocin affects other systems of the body i.e. it possesses renal, hepatic and gonadal toxicities. It is recommended to study the other organs at this dose for a comprehensive evaluation.
ACKNOWLEDGMENTS We thank the Department of Anatomy and the management of Mahatma Gandhi Medical College and Research Institute for providing facilities for animal studies and technical support.
REFERENCES 1. Thomas CC, Philipson LH. Update on Diabetes Classification. Vol. 99, Medical Clinics of North America. 2015. p. 1–16. 2. Drouin P, Blickle JF, Charbonnel B, Eschwege E, Guillausseau PJ, Plouin PF, et al. Diagnosis and classification of diabetes mellitus. Diabetes Care . 2009;32. 3. Nathan DM. Long-Term Complications of Diabetes Mellitus. N Engl J Med. 1993;328(23):1676–85. 4. Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: Evolving strategies for the treatment of patients with type 2 diabetes. Vol. 60, Metabolism: Clinical and Experimental. 2011. p. 1–23. 5. Wu J, Yan LJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic cell glucotoxicity. Diabetes, Metab Syndr Obes Targets Ther. 2015;8:181–8. 6. Raza H, John A. Streptozotocin-induced cytotoxicity, oxidative stress and mitochondrial dysfunction in human hepatoma HepG2 cells. Int J Mol Sci. 2012;13(5):5751–67. 7. Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22(4):359–70. 8. Ventura-Sobrevilla J, Boone-Villa VD, Aguilar CN, Román-Ramos R, Vega-Ávila E, Campos-Sepúlveda E, et al. Effect of varying dose and administration of streptozotocin on blood sugar in male CD1 mice. Proc West Pharmacol Soc. 2011;54:5–9. 9. Turner P V., Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: Routes of administration and factors to consider. Vol. 50, Journal of the American Association for Laboratory Animal Science. 2011. p. 600–13. 10. Fröde TS, Medeiros YS. Animal models to test drugs with potential antidiabetic activity. Vol. 115, Journal of Ethnopharmacology. 2008. p. 173–83. 11. Rajesh, R, Arunchandra Singh S, Anandraj Vaithy K, Manimekalai K, Kotasthane D, Rajasekar SS. The effect of mucuna pruriens seed extract on pancreas and liver of diabetic wistar rats. Int J Cur Res Rev. 2016;8(4). 12. Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125(3):451–72. 13. Rajesh R, Kotasthane DS, K M, Singh A, V S, Rajasekar SS. Histopathological and Histomorphometric analysis of Pancreas and liver of diabetic rats treated with MucunaPruriens seed extract. Ann Pathol Lab Med. 2017 Oct;4(5):A546–52. 14. Ogbonnaya EC, Eleazu KC, Chukwuma S, Essien UN. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord. 2013;12(1):60. 15. Suresh S, Prithiviraj E, Prakash S. Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl. 2010;33(1):22–32. 16. Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59(7):365–73. 17. Sefi M, Fetoui H, Lachkar N, Tahraoui A, Lyoussi B, Boudawara T, et al. Centaurium erythrea (Gentianaceae) leaf extract alleviates streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. J Ethnopharmacol. 2011;135(2):243–50. 18. Etuk EU. Animals models for studying diabetes mellitus Department of Pharmacology , College of Health Sciences , Usmanu Danfodiyo University ,. Agric Biol J North Am. 2010;1(2):130–4. 19. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care [Internet]. 2014;37:S81-90. 20. Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, et al. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Vol. 45, Laboratory Animals. 2011. p. 131–40. 21. Islam S, Loots DT. Experimental rodent models of Type 2 Diabetes: A Review. Methods Find Exp Clin Pharmacol. 2009;31(3):1–13. 22. Suresh S, Prakash S. Effect of Mucuna pruriens (Linn.) on Sexual Behavior and Sperm Parameters in Streptozotocin-Induced Diabetic Male Rat. J Sex Med. 2012;9(12):3066–78.
Authors who publish with MedPulse International Journal of Anatomy (Print ISSN: 2550-7621) (Online ISSN: 2636-4557) agree to the following terms: Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal. Authors are permitted and encouraged to post links to their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home
