|
Table of Content Volume 13 Issue 2 - February 2020
Protective effects of Coconut(Cocos Nucifera Linn) shell extract on the microstructural and functional abnormalities of kidneys in rats with metabolic syndrome
Sreekala V1, Rajesh R2*
1PhD Scholar, Department of Dravyaguna, Banaras Hindu University, Varanasi, Uttar Pradesh, INDIA. 2Assistant Professor, Department of Anatomy, KVG Medical College, Sullia, Karnataka, INDIA. Email: rajesh2361@gmail.com
Abstract Background: Renal dysfunctions associated with metabolic syndrome lead to the development of chronic kidney disorders. Folklore medicine claims that water boiled with crushed pieces of dried coconut shell, possesses hypocholestremic, hypoglycemic and anti-obesity properties. In this regard it is important to evaluate whether the drug possess any protective or toxic effects on vital organs like kidneys on long term administration. The study was designed to assess the nephroprotective effect of dried coconut shell aqueous extract Wistar albino rats with high fructose induced metabolic syndrome. Metabolic syndrome is induced in adult male Wistar albino rats by giving high fructose diet (55%) and Fructose enriched water (15%) for 75 days. Dried coconut shell aqueous extract was given at a dose of 200 mg /kg b.w and 400 mg/kg b.w along with High Fructose Diet (HFD) for 75 days. The extent of renal damage was assessed by estimations of serum urea, uric acid, serum creatinine and histological studies. HFD produced a significant increase in serum creatinine, urea and uric acid levels. Histological sections of kidneys presented with fatty changes, mesangial expansion, inflammation and tubular necrosis. Administration of dried coconut shell aqueous extract (200 mg/kg) significantly restored renal functions and ameliorated microstructural alterations, whereas 400 mg/ kg b.w treatment failed to produce any significant nephroprotective effect. It is concluded that the active ingredients of the coconut shell are effective in controlling renal dysfunctions associated with metabolic syndrome. Key words: Coconut shell, Dyslipidaemia, High Fructose Diet, Metabolic Syndrome.
INTRODUCTION Metabolic syndrome (Met S) is a cluster of abnormalities characterized by obesity, insulin resistance, hypertension and dyslipidemia.1 Lifestyle disorders including metabolic syndromeplay a crucial role in the development of microvascular complications that lead to kidneydysfunctions2 .There are several reports on renal dysfunctions associated with metabolic syndrome leading to the development of Chronic kidney disorders (CKD) 3,4, which is the main cause of end stage kidney failure, a major health problem in developing countries. The 2019 National Chronic Kidney Disease (CKD) Fact Sheet states that more than 15% of US adults i.e., 37 million people are suffering from CKD5. CKD is associated with a range of complex derangements in physiological and metabolic functions, such as uremia, metabolic acidosis, abnormalities in lipid metabolism, Insulin resistance, Inflammation, Oxidative stress and Anaemia6 Worldwide prevalence of Lifestyle disorders, a major risk factor of metabolic syndrome, is increasing steadfast. In humans, dietary changes including increased consumption of fructose, primarily from sucrose (a disaccharide consisting of 50% fructose) and high-fructose corn syrup (HFCS; 55–90% fructose content), plays a major role in the development of Metabolic syndrome7. Fructose administration in rats probably induces variable degrees of Met S that may result in different physiological and morphological renal responses8. The associated Oxidative stress and hypertension in Met S, are the other risk factors in chronic kidney diseases9. Animal studies suggest that high fructose diet induced metabolic syndrome, can produce micro structural and functional alterations in Kidney10. Currently available medications are not ensuring a comprehensive cure for the related complications of metabolic syndrome. Moreover, long term use of these drugs are associated with side effects to internal organs like Kidney11,12. A drug therapy that normalizes the metabolic dysfunctions should be able to prevent, delay or considerably reduce the severity of these complications. There is a folklore claim existing in Kerala regarding the efficacy of water boiled with crushed pieces of dried ripe coconut shell in the management of obesity, hyperlipidemia and hyperglycemia. From the previous reports, Cocos nucifera endocarp was found to have significant antihypertensive and vasorelaxant properties in hypertensive rats13.Apart from this, it has antioxidant, anti-inflammatory and antimicrobial properties 14. But no study has been reported on its possible nephroprotective effects. So, this study was designed to explore the protective effects of coconut shell (Cocos nucifera Linn.) against the microstructural and functional damages of Kidney in high fructose induced metabolic syndrome in wistar rats.
MATERIALS AND METHODS2.1. Test drug Ripe coconut shells (Cocos nucifera Linn.) were procured from Calicut, Kerala. The shells were and dried in sunlight,the outer fibers were scraped off, then crushed into small pieces and pulverized to a coarse powder. This was used for the preparation of aqueous extract as per Ayurvedic Pharmacopoeia of India. 2.2. Preliminary phytochemical studies Preliminary phytochemical studies are conducted as per standard procedures 15, 16. Experimental studyThe study protocol was approved by the institutional Animal Ethics Committee, SDM Centre for Research in Ayurveda and Allied sciences, Udupi, (CPCSEA/IAEC/15-16-KT.20). The animal handling was performed according to Good Laboratory Practice 17. AnimalsAdult male Wistar albino rats, weighing 180 - 240 g were procured from the Central animal house of the SDM Centre for Research in Ayurveda and Allied sciences. The rats were housed under standard conditions of temperature (22 ± 2°C), humidity and 12 hr light /dark cycle, with free access to standard pellet diet (Sai Durga Feeds, Bangalore) and water ad libitum. The animals were acclimated to the standard experimental conditions for seven days before the experimental study. Acute oral toxicity studiesAcute oral toxicity studies were conducted as per internationally accepted protocol drawn under OECD guidelines 42518. Induction of metabolic syndromeHigh fructose diet (55%) and fructose enriched water (15 %) were administered for 75 days to induce metabolic syndrome in animals, characterized by Obesity, hyperglycemia and hyperlipidemia19. 2.3.4. Experimental design: Rats were divided into four groups with six animals in each. In group I, rats were fed with standard diet and water, and kept as normal control. In group 2, rats were fed with High Fructose diet and fructose enriched water, and kept as positive control. Group III and IV received test drug 200mg/kg (Therapeutically Effective Dose) and 400 mg/kg (Double the Therapeutically Effective Dose) respectively, along with high fructose diet and fructose enriched water for 75 days. Assessment of metabolic parametersDaily Assessment of Food and water intake and weekly assessment of body weight changes were done. Once a week, the animals were placed individually in metabolic cages and urine was collected from 24 hr sample. Blood Collection and biochemical analysisAfter 75 days of treatment, animals were kept for overnight fasting. The blood sample was collected by retro-orbital puncture under light ether anaesthesia.The samples were collected separately into sterilized, dry centrifugation tubes containing sodium citrate 0.01%,and allowed to stand for 30 min at 37º C. The clear serum was separated at 2500 rpm for 10 min using micro centrifuge. Creatinine, urea, and uric acid were measured using a fully automated clinical analyzer (ERBA-EM-200) and commercial kits from Erba diagnostics, according to the standard protocols provided by manufacturers. Histological studiesAfter the blood collection, the animals were sacrificed by painless cervical dislocation method. Animals were dissected; kidneys were identified and observed for gross appearance and colour change with magnifying glass. The kidneys were dissected out; extraneous tissues are cleaned after placing it in a Petri dish containing normal saline. The tissues were carefully wiped with a tissue paper and weighed by using a digital weighing machine. Kidney tissues were immediately transferred to 10 % formalin for paraffin embedding and stained with Haematoxylin and Eosin (H&E). Statistical analysisAll the data are expressed as Mean ± SD. Statistical analyses were performed by one way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Probability values less than 0.05 were considered significant. All analyses were performed using Graph Pad Instat (Graph Pad Software, San Diego, CA, USA). 3.Observations and Results 3.1. Acute toxicity studiesIn acute toxicity study, the aqueous extracts of dried ripe coconut shells did not show mortality at 2000 mg/kg b.w dose. Therefore 2000 mg/kg dose was considered as LD50 cutoff the dose under GHS 5 (safe dose), as per Globally Harmonized Classification System (GHS) for Chemical Substances and Mixtures described in OECD guidelines 423. Hence, 200 mg/kg (low) and 400 mg/kg (high) doses were selected for in-vivo therapeutic study. 3.2. Weight of kidneysIncreased weight gain was obvious in both experimental and positive control group. There was a significant difference between the two control groups as regards body weight (p 0.0001). Increased kidney weight was observed among the untreated rats. Among control groups, kidney weight appeared statistically significant. Though TG2 group showed higher kidney weight, no statistical significance was observed with TG1. Table 1
All the values were expressed in Mean ± SD, different alphabets between groups denotes significance at p < 0.05, a - PC compared with NC; b - TG compared with PC, c – TG1 compared with TG2. 3.3. Effect on Kidney profileTable 2 shows the Serum urea, uric acid and creatinine levels of control and experimental rats after 75 days of treatment. Serum urea levels of the positive control rats showed minimal and statistically insignificant increase. The treatment groups showed a minimal increase and when comparing both, no statistical significance was observed. Serum uric acid and creatinine levels were significantly increased in positive control group. Treatment with the coconut shell extract resulted in significant reduction in serum uric acid and creatinine but comparison among the treatment group showed no statistical significance. When compared with the positive control, serum creatinine levels were significantly lower in treatment groups, whereas no statistical significance was seen in serum uric acid level.
Table 2: The effect of aqueous extract of Coconut shell on Serum urea, Uric acid and Creatinine
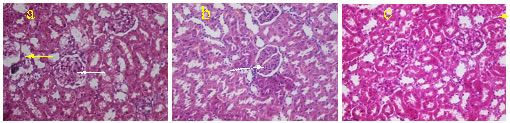
All the values were expressed in Mean ± SD, different alphabets between groups denotes significance at p < 0.05, a - PC compared with NC; b - TG compared with PC;c – TG1 compared with TG2. 3.4. Histological studiesSome of the sections from the positive control group showed pronounce lymphatic infiltration. Among these, few of the glomeruli appeared distorted and slightly expanded. The proximal convoluted tubules showed tubular necrosis along with loss of brush border. Some of the glomeruli showed foci of hyalinised eosinophilic material in the extracellular mesangium causing mesangial expansion. Hyaline changes were evident near the vascular poles. Glomerular membrane also appeared to be affected. In The TGF group, when compared with the positive control, lymphatic infiltrations were considerably less, only a few of the glomerulus showed mild hyalinisation. The PCT showed mild epithelial damage, vascular poles and glomerular membrane appeared normal. Similar findings were seen in TG II also.
Figure 1. H&E stained sections of kidneys (a). Positive control showing tubular destruction (yellow arrow), hyalinization (white arrow) and mesangial expansion. (b and c.) TG1 and TG2 showing mild lymphatic infiltration (White arrow) with minimal tubular damage (Yellow arrow). DISCUSSIONDietary fructose plays a pivotal role in the development of metabolic diseases. This is mainly due to the continuous increase in fructose (and the total added artificial sweetener consumption), and by the increase of high-fructose corn syrup (HFCS) use. The Metabolic syndrome is a combination of abnormalities characterized by obesity, insulin resistance and dyslipidemia20. The association between the metabolic syndrome and kidney disease has been studied by several researchers21,22. The possible mechanisms of renal injury include insulin resistance and oxidative stress, increased proinflammatory cytokine production, increased connective tissue growth and profibrotic factor production, increased microvascular injury, and renal ischemia 23. The combination of high fructose diet (HFD) and fructose water (FW)is acclaimed as a standard model for the development of metabolic syndrome that is similar to the human type 24,25. In the present study, animals developed metabolic syndrome with HFD + FW treatment. The increased weight of the kidneys in our study suggested that HFD + FW treatment is effective in elucidating nephrotoxicity. Previous researchers also put forwarded similar findings in HFD rats 19,25. The Treatment with the test drug effectively reduced the weight gain, which was more evident in TG1 than TG2. In the present study, the HFD + FW fed rats showed increased serum urea, creatinine and uric acid levels. These are lowered in the test drug groups. Among this, serum creatinine level were significantly decreased in TG1 when compared with positive control. This might be due to the presence of tannins and rich bioactive fibre content 26. Consumption of fiber can enhance the energy substrate available to fecal bacteria, that in turn stimulates their proliferation, andcould reduce serum urea by excretion of accumulated nitrogenous wastes via fecal matter 27. Yokozawa T, et al reported that green tea tannins prevent proliferation of mesangial cells and helps in improving the regulation of glomerular filtration rate 28. The histological findings from the present study further supports the kidney profile. The typical features of the CKD such as interstitial inflammation, tubular atrophy and mesangial expansion were evident in this study 10. Results of the study indicated that dried coconut shell extract significantly ameliorated the microstructural changes in the kidney.
CONCLUSIONThe study showed that dried ripe coconut shells exerted renoprotective effects by ameliorating the structural and functional abnormalities of kidneys by high fructose induced metabolic syndrome in therapeutically effective dose. This indicates that dried coconut shells possess potential as a new therapeutic substance, although further studies are warranted. ACKNOWLEDGEMENT We thank the management and the research officers of SDM Centre for Research in Ayurveda and Allied science for providingtechnical supports and valuable guidance. REFERENCES
Authors who publish with MedPulse International Journal of Anatomy (Print ISSN: 2550-7621) (Online ISSN: 2636-4557) agree to the following terms: Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal. Authors are permitted and encouraged to post links to their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
|
|
 Home
Home