|
Table of Content Volume 17 Issue 3 - March 2021
Genomics of Acetylcholinesterase gene expression and related RNA content in brain of five different vertebrate species: A prospective institutional study
Rajesh Bangaraiahgari1, Ramesh Bangaraiahgari2, Rafi Md3, Rajkiranreddy B4, Ramakanth Bhargav Panchangam5*
1Associate Professor, Department of Anatomy, Surabhi Institute of Medical Sciences, Telangana, India. 2Associate Professor, Department of Biochemistry, Surabhi Institute of Medical Sciences, Telangana, India. 3Professor, Department of Biochemistry, Surabhi Institute of Medical Sciences, Telangana, India. 4Research scientist, Smart, Sunshine Hospital, Secunderabad -500003, Telangana, India. 5Consultant Endocrine and Metabolic Surgeon,Associate Professor, Surgical Endocrinology,Endocare Hospital, Vijayawada, AP, INDIA. Email:endoanswers@gmail.com
Abstract Background:Inhibition of acetylcholinesterase (AChE), the metabolizing enzyme of acetylcholine is evolving as the most important therapeutic target for development of cognitive enhancers. However, AChE activity in brain has not been properly evaluated on the basis of sex. In the present study, AChE expression was investigated in different brain areas of cerebrum and cerebellum in male and female of five vertebrate species. On comparing male and female genders, increased AChE activity was seen in cerebrum and cerebellum of female of five vertebrate species. However, no significant change in AChE activity was found between cerebrum and cerebellum within the same male and female. Thus it appears that sex alters AChE activity in different brain regions (G4 isoform) that may vary in male and female. Sequence analysis revealed that highest divergence was found in between male cerebrum and female cerebrum and least divergence was found in between male cerebellum and female cerebellum with control AChE JX 190065,1, HM.998937.1, EF534897.1, NM_205418.1, NM_172009.1. Key Words: Acetylcholinesterase; Physiology; Invertebrates; Vertebrates; Cerebrum
INTRODUCTION Acetylcholinesterase (AChE) is one of the most efficient enzymes of nervous system which is concentrated at the cholinergic synapses and at neuromuscular synapses where it rapidly hydrolyses the neurotransmitter acetylcholine (ACh) in to choline and acetate thus playing a vital role in cholinergic neurotransmission. The term acetylcholinesterase was introduced in 1949 by Augustintion and Nachmansohn for specific cholinesterase capable of hydrolyzing acetylcholine faster than other esterases. In 1964 the commission of enzymology recommended the name “Acetylcholinesterase” (Acetylcholine Acetyl hydrolase; 3.1.1.7) for a true and specific cholinesterase. The distribution of the enzyme in the central and peripheral nerve tissues of different vertebrates demonstrates a high range of variation.1,2 It has been noted to be localized in non neuronal tissues and glial cells also.3 The enzyme also exhibits molecular diversity with its six different molecular forms and structural dynamics, which facilitates its affinity and action with various legends.4 In addition, AChE is considered to play several non classical roles independent of its catalytic function i.e. hydrolysis of ACh. These classical and non classical roles of AChE illustrate adequacy about its wide occurrence in neuronal and non neuronal tissues. [5,6]AChE is widely distributed; it occurs in the central (CNS) and peripheral nervous systems (PNS), and the motor end-plates of the skeletal muscle and the electric organ, but it is also found in many other tissues and in erythrocytes. Therefore such a wide distribution and various functions, molecular forms, structural dynamics etc. of AChE provide adequate base to recall it a versatile enzyme, a detailed knowledge of which, might help to design specific drugs to combat various neurodegenerative diseases associated with this enzyme. In this context, we set up to study the distribution, structure of AChE gene and related RNA in neuronal tissues of vertebrate animals.
MATERIAL AND METHODS This prospective study was conducted in Anatomy department of a tertiary care teaching medical school in South India. The study was approved by institutional ethical committee. We ensured that study complied with biomedical ethics guidelines for animal experimentation as laid down by Indian council of Medical Research (ICMR). AChE cDNA sequence in neuronal tissues of five vertebrate species (Channa striata, Duttaphrynusmelanostictus Hemidactylus frenatus, Gallus gallusdomesticus, Rattus norvegicus) were analysed in this study. The male and female species of five representative vertebrate species were purchased from a local supplier and transported live to the laboratory in aerated tanks. During the acclimatization period, they were fed daily (Safe feed 7711, Charoen Pokphand Foods PCL, Thailand) weighing about 1% of the body weight, and were then fasted for 24 h before the experiment. They were sacrificed, the brain was rapidly removed, weighed, and dissected for RNA extraction and sequencing the brain was rapidly removed for RNA extraction followed by reverse transcription and fold induction of gene expression between AChE and 28S rRNA genes, and were then analysized by PCR and Gel analysis.
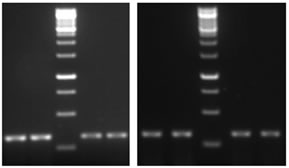
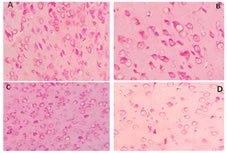
Total RNA isolation Total RNA was extracted from the brain of Duttaphrynusmelanostictus using RNeasy Mini Kit (QIAGEN GmbH, Germany),vaccording to the manufacturer’s instructions. RNA was analyzed in 1% agarose gel, containing ethidiumbromide and visualized with UV light. The 1 Kb DNA ladder plus and 100 bp DNA ladder plus (Fermentas, USA) was used as molecular marker. AChE cDNA synthesis and Sequence Analysis Reverse transcription-polymerase chain reaction (RT-PCR): Complementary DNA (cDNA) was synthesized by using First Strand cDNA Synthesis kit for PCR thermo scientific, according to the manufacturer’s instructions. PCR amplification used degenerate primers. Primers of AChE gene designed in conserved region of all five vertebrates from GENBANK using CODEHOP program. Entire set of primers used are displayed in Table 1. For the PCR reaction, 4 Hl of cDNA from each synthesis were added to 7 Hl of ‘2X PCR master mix’ containing 10X PCR buffer, 10 mM dNTP, 25 mM MgCl2, 5 U of Taq DNA polymerase (Fermentas, USA). Twenty HM of each pair of the primers was added, and the final volume was adjusted to 14 Hl with nuclease free water. The mixtures were denatured at 94oC for 3 min. Thirty five cycles of PCR were carried out, with denaturation at 94oC for 45 sec, annealing at 57oC for 30 sec, and extension at 72oC for 1 min, followed by a final extension period of 5 min. PCR products were analyzed by electrophoresis on 1% agarose gels stained with GelStar Nucleic Acid Gel Stain (Cambrex Bio Science Rockland, Inc.). Representative illustrations of electrophoresis are shown in Fig. 1. PCR products were cloned into the pGEMT plasmid vector (Promega) and sequenced using forward and reverse primers. Sequencing was performed with the Big DyeTM Terminator Cycle Sequencing Ready Kit, version 3.0 (ABI PrismTM, Perkin Elmer) and an ABI 3700 Applied Biosystems Model automated DNA sequencer. Nucleotide sequences of NWS were analyzed by BLASTN to search for similarities, and sequence alignments was performed with CLUSTAL W (Megalign program, DNASTAR Inc., Madison, WI). Quantitative assessment of RNA by methyl green-pyronin staining The tissues are fixed in methacorn fixatives for 4 hours for fixation, and then followed by routine histological processing. The 5 to 6 um sections are taken for all tissues and stained with MGP (methyl green pyronin). The Number of RNA granules are estimated by image analysis by used software IMAGE pro 6.2. Related illustrations are shown in Figs. 2 and 3.
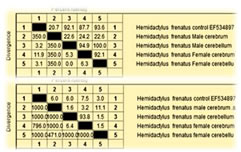
RESULTS AChE cDNA sequence was used to investigate difference in male and female cerebrum and cerebellum. The ORF of AChE is comprised of 338 nucleotides (GenBank accession number JX190065.1), showing significant nucleotide similarity 88.2% and 83.4% respectively with Channa striata male cerebrum and cerebellum whereas 88.1 % with female cerebrum and cerebellum AChE. AChE cDNA sequence was used to investigate difference in male and female cerebrum and cerebellum. The ORF of AChE is comprised of 221 nucleotides (GenBank accession number HM998937.1), showing significant nucleotide similarity 22.2% and 99.1% respectively with Duttaphrynusmelanostictus male cerebrum and cerebellum whereas 14.9 % and 89.6 respectively with female cerebrum and cerebellum AChE. Representative sequence is shown in Fig. 4.AChE cDNA sequence was used to investigate difference in male and female cerebrum and cerebellum. The ORF of AChE is comprised of 256 nucleotides (GenBank accession number EF534897), showing significant nucleotide similarity 86.2% and 84.4% respectively with Hemidactylus frenatus male cerebrum and cerebellum whereas 87.1 % with female cerebrum and cerebellum AChE. AChE cDNA sequence was used to investigate difference in male and female cerebrum and cerebellum. The ORF of AChE is comprised of 359 nucleotides (GenBank accession number NM_205418.1), showing significant nucleotide similarity 89.2% and 88.4% respectively with Gallus gallusdomesticus male cerebrum and cerebellum whereas 87.1 % with female cerebrum and cerebellum AChE. Representative illustration is shown in Fig. 5.AChE cDNA sequence was used to investigate difference in male and female cerebrum and cerebellum. The ORF of AChE is comprised of 543 nucleotides (GenBank accession number NM_172009.1), showing significant nucleotide similarity 82.2% and 81.4% respectively with Rattus norvegicus male cerebrum and cerebellum whereas 84.1 % with female cerebrum and cerebellum AChE. The highly divergent regions between the AChE sequences when compare with standard sequence are found in male cerebrum where as when compare with male cerebellum, female cerebrum, female cerebellum was less significant, But when compare with male and female cerebrum and cerebellum, the highest divergence was found in between male cerebrum and female cerebrum and least divergence was found in between male cerebellum and female cerebellum. Expression of AChE gene Expression of Acetylcholinesterase (AChE) was significantly more in female cerebrum and cerebellum when compared with male cerebrum and cerebellum. Also, it was found that methyl green-pyronin staining on Channa striata,Duttaphrynusmelanostictus, Hemidactylus frenatus, Gallus gallusdomesticus and Rattus norvegicus, brain regions shows more RNA granules in female cerebrum and female cerebellum. Figure 1: Electrophoretogram of AChE – A) Lane 1,2 Female cerebrum; Lane 3, 4 Male cerebrum, B) Lane 1, 2 Female cerebellum; Lane 3, 4 Male Cerebellum. The PCR product size is 338 base pairs Figure 2: Methyl green-pyronin staining on Channa striata brain regions (A) Female Cerebrum showing maximum numbers of RNA granules in comparison with male cerebrum (B) where as again RNA granules are more in female cerebellum (C) than that seen with male cerebellum (D) Figure 3: Methyl green-pyronin staining on Duttaphyrus melanosticus brain regions (A) Female Cerebrum showing maximum numbers of RNA granules in comparison with male cerebrum (B) whereas again RNA granules are more in female cerebellum (C) than that seen with male cerebellum (D)
Figure 4: Alignment of nucleotide sequences of Channa striata from male and female cerebrum and cerebellum with ACHE JX 190065.1
Figure 5: Percentage identity and divergence of Hemidactylus frenatusAChE nucleotide and amino acid sequences from and Hemidactylus frentusAChE EF534897 of male and female cerebrum and cerebellum
Table 1: Primer sequences and their size
DISCUSSION Acetylcholinesterase (AChE) terminates the neurotransmission at cholinergic synapses by splitting the neurotransmitter acetylcholine. The nature and distribution of the enzyme has extensively been studied in many invertebrates and vertebrates including human, histochemically and biochemically. The distribution of the enzyme in the central and peripheral nerve tissues of different vertebrates demonstrates a high range of variation.7 It has been noted to be localized in non neuronal tissues and glial cells also. The enzyme also exhibits molecular diversity with its six different molecular forms and structural dynamics which facilitates its affinity and action with various legends.8 In addition, AChE is considered to play several non classical roles independent of its catalytic function i.e. hydrolysis of Ach. These classical and non classical roles of AChE illustrate adequacy about its wide occurrence in neuronal and non neuronal tissues.6 The importance of AChE in body homeostasis is underscored by the fact that they are the targets of some of the most potent toxins including insecticides, snake venom and chemical weapons.6AChEs have been so far identified in different tissues of most vertebrates and more than 20 invertebrate animals.9,10 For instance, AChE activity has been detected in erythroid cells, brain, muscle, liver, kidney and lungs of vertebrates. It was also detectable in different tissues of invertebrates, such as in the gills, mantle and haemolymph of mollusk.11,12 There is a great difference in the amino acid sequence of AChEs from different animal, and it even varies greatly among the different tissues of the same organism. All the AChEs share some conserved structural features responsible for their catalysis function. For example, an active site triad (Ser, Glu and His) exist in all the reported AChEs, and the three residues form a plannar array at the bottom of a deep and narrow gorge, which closely resembles the catalytic triad of other a/b hydrolase fold family proteins.13Acetylcholinesterase (AChE; EC 3.1.1.7) in vertebrates was involved in cell development and maturation, neuronal development and nerve regeneration and inflammation modulation. AChE had also been identified in most invertebrates, including molluscs, arthropods, platyhelminthes, annelida and nematoda. And AChE was also reported to be involved in many behaviors in these invertebrates, including locomotion, reproduction, egg laying, male mating, embryo development and digestive activity. However, the immunomodulation of AChE is still unclear in invertebrates. In the present study, an AChE gene was studied in male and female cerebrum and cerebellum of five vertebrates (Ratus norvegicus, Gallus-gallusdomesticus, Hemidactylus frenatus, Duttaphrynusmelanostictus, Channa striata). An AChE gene was studied in male and female Ratus norvegicuscerebrum and cerebellum. The deduced protein of Ratus norvegicusAChE (NM_172009.1) was comprised of 160 amino acids,and it shares 49.4% and 11% identity with other AChEs of cerebrum of male and female cerebrum respectively, whereas if we see male and female cerebellum we found very less identity 8.1 and 10% respectively. The ORF of AChE is comprised of 112 aminoacids (GenBank accession number NM_205418.1), showing significant aminoacids similarity 90.2% and 92.0% respectively with Gallus-gallus domesticus male cerebrum and cerebellum whereas 92% and 94% with female cerebrum and cerebellum AChE. Other vertebrate Hemidactylus frenatus was also studied and deduced protein of its AChE (EF534897) was composed of 203 amino acids and it share 20% and 87% with male and female respectively where as in cerebellum its show significant match 92.1% and 93.6% with male and female respectively. The ORF of AChE is comprised of 73 aminoacids (GenBank accession number HM998937.1), showing significant nucleotide similarity 5.5% and 4.1% respectively with Duttaphrynusmelanostictus male cerebrum and cerebellum whereas 2.7% and 90.4%respectively with female cerebrum and cerebellum AChE.Channa striata gene was studied in same line and deduced protein of AChE (JX190065.1) was composed of 253 amino acids, and it shared 4.3 and 8.7 %with male and female cerebrum respectively and cerebellum shows low identity 6.7 and 4.3 with male and female cerebellum respectively.In the present study, AChE activity was investigated in different brain areas of cerebrum and cerebellum in male and female of different vertebrates. Females had a significant increase in AChE activity in cerebrum and cerebellum in comparison with male cerebrum and cerebellum. We also found that RNA granules are more in female cerebrum and cerebellum and it may be one of the reason that affects the expression of AChE gene. On the basis of this new understanding of AChE brain organization and its evolutionary relationships, we found that female had more AChE activity or more expression than male but the exact reason was not clear. Therefore more studies are required to know why AChE activity is more in female counter part.Restraint of acetylcholinesterase (AChE), metabolizing compound of acetylcholine, is in a matter of seconds the most imperative restorative focus for improvement of psychological enhancers. Nonetheless, AChE movement in cerebrum has not been legitimately assessed on the premise of sex. It is unrealistic, at present, to dole out a positive component to clarify the example of deficiency saw in the chemical movement in male. Among the conceivable outcomes, diminished blood stream in cerebrum bringing about hypoxia has been proposed for decrement in AChE turnover in entire mind of male. Absence of consistency in profile of AChE movement might be an impression of useful heterogeneity in focal cholinergic framework saw by a few specialists on different parameter.
CONCLUSIONS Variable enzyme activity in different brain areas and sex related changes was observed in Duttaphrynusmelanostictus, might be having an important bearing for functional heterogeneity reported for central cholinergic neuronal system and development of specific pharmacotherapy for cognitive disorders.
REFERENCES
Policy for Articles with Open Access: Authors who publish with MedPulse International Journal of Community Medicine (Print ISSN: 2579-0862) (Online ISSN: 2636-4743) agree to the following terms: Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal. Authors are permitted and encouraged to post links to their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
|
|
 Home
Home