|
Table of Content - Volume 18 Issue 1 - April 2021
Rajesh Bangaraiahgari1, Ramesh Bangaraiahgari2, Rafi Md3, Rajkiran reddy B4, Ramakanth Bhargav Panchangam5*
1Associate Professor, Department of Anatomy, Surabhi Institute of Medical Sciences, Telangana, India. 2Associate Professor, Department of Biochemistry, Surabhi Institute of Medical Sciences, Telangana, India. 3Professor, Department of Biochemistry, Surabhi Institute of Medical Sciences, Telangana, India. 4Research Scientist, Smart, Sunshine Hospital, Secunderabad -500003, Telangana, India. 5Consultant Endocrine and Metabolic Surgeon, Associate Professor, Surgical Endocrinology,Endocare Hospital, Vijayawada, AP, INDIA. Email:endoanswers@gmail.com
Abstract Background: Due to advancement in the tools for the tissue-analysis by structural and functional genomics, proteomics and metabolomics, there is a constant demand in the area of handling and preserving test material with intact and extractable messenger RNA has become mandatory. To find an optimal fixative for tissues aimed for RNA-maintaining abilities of 2 precipitating tissue fixatives, such as Carnoys, RNA laterand Methacarn solution. These fixatives preserved the morphology and total RNA that was of significantly higher quality than RNA extracted from formalin-fixed tissue. Methacarn fixative performed better than RNAlater and Carnoysin maintaining the integrity of RNA, especially when the fixed, paraffin-embedded tissue blocks were stored at room temperature for more than 3 months. Total RNA extracted from microdissectedbrains of all vertebrates. The emerging role of fixatives in research, and in clinical work in the near future will be to stabilize and preserve RNA in the biological specimens and to replace formalin as the vertebrate tissues. According to our data, methacarn fixative is an excellent candidate that can be used as a fixing reagent for vertebrate samples. Keywords: Fixatives, vertebrates, Methacarn solution, Carnoy’s solution, RNA Later, Brain
INTRODUCTION Paraffin embedding of tissue has been routinely used in slide preparation as it provides a convenient way for handling tissues and its subsequent staining to study its morphology. Until recently, 10% neutral buffered formalin was extensively used for fixation of fresh tissues to produce paraffin-embedded tissue blocks.1-5 Although formalin preserves morphological architecture and is cost effective, its cross-linking components lead to RNA chemical alterations and fragmentation, impairing quantification of gene expression.6,7 The gold standard for molecular study is still unfixed or frozen tissues. However, these techniques cannot be applied to the field of pathology as they do not preserve precise morphological features and can impair histological diagnosis. 4, 8In order circumvent this problem, many attempts have been made to develop a fixation method that can preserve the histological structure of tissues, without destruction of RNA.1,4,9–12 Aldehydes are well known to be good ultrastructural preservers via the inter- and intra-molecular cross-linking of amino and aldehyde groups.13 Different forms of alcohol-based fixatives have been developed as formalin substitutes, such as Methacarn and Carnoy’s solution. Methacarn and Carnoy’s solution are commonly used during fixation of nucleic acid. These fixatives are non-cross-linking organic solvents that have been shown to maintain tissue morphology and preserve RNA and DNA integrity as they minimize chemical modifications.1,14,15 Although, these alcohol-based fixatives are known to be superior to formalin in preserving RNA, their effectiveness in maintaining the histological structure samples – especially in regards to their ability to facilitate an accurate reading of pathological findings in human tissues – has not yet been fully established.10,16,17The recent development of the laser capture microdissection technique has enabled us to obtain pure cell populations in order to determine specific gene expression patterns in tissues and to link the genetic changes with the pathological observations.8,15,17–20 For the microdissection of tissues, the preservation of histomorphology and molecular structures is essential. Therefore, tissue embedding after fixation is preferable for micro dissected tissue preparation if high yield and quality of molecules can be guaranteed. As micro dissection limits the yield of molecules, extraction efficiency and quality of molecules are critical for analysis in microdissected cells. For this reason, choosing fixatives that leave RNA intact is very important. Fixatives other than formalin (e.g. alcohol-based fixatives) are well known to be more effective in preserving RNA, but few studies have looked at whetherthese fixatives are also effective in maintaining histomorphologic structure. Especially rear are studies the effects of fixative choice for human tissue preparation for laser capture microdissection. In this study we investigate the best fixative for use in the preparation of human tissues for laser capture microdissection, and for the preservation of RNA molecules and histomorphologic structure.
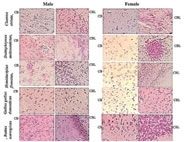
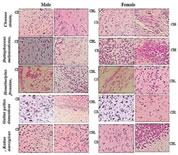
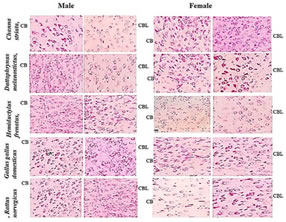
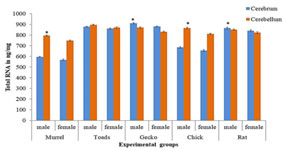
MATERIALS AND METHODS Tissue collection and fixation This prospective study was conducted in Anatomy department of a tertiary care teaching medical school in South India. The study was approved by institutional ethical committee. We ensured that study complied with biomedical ethics guidelines for animal experimentation as laid down by Indian council of Medical Research (ICMR). All five vertebrate species ofmale and female weighing an average 108 ± 26 (90 -180 grams) were purchased from a local supplier and transported live to the laboratory in aerated tanks. During the acclimatization period, the animals were fed daily (Safe feed 7711, Charoen Pokphand Foods PCL, Thailand) weighing about 1% of the body weight, and were then fasted for 24 hours before the experiment. They were sacrificed, the brain was rapidly removed, weighed, dissected and samples were fixed for 12 h at 40C inMethacarn (MC)solution (methanol: chloroform: glacial aceticacid=6:3:1 (v/v)), Carnoy’s solution (CS), ethanol: chloroform: glacialacetic acid = 6:3:1 (v/v)) and RNA later (RL) and then paraffin embedded using standard procedures. For assessment of the morphological preservation abilityaccording the fixative used, methyl-green pyronin slides were madeusing samples from all paraffin-embedded tissue blocks. Objectives of this study are - 1. Which fixative provides a better preservation of RNA granules in the tissues and enables to understand its evolutionary process and 2. Quantifying the amount of RNA granules in CB and CBL form lower to higher vertebrates of both male and female species (i.e. Fish, toad, lizard, chick, and rat) for comparing and understanding the evolutionary mechanism in them. RESULTS The Table 1 compares the efficiencies of varied fixatives in stabilizing the RNA content in the brains of male and female Chenna straita. The tissues which were fixed with Methacarn showed more number of RNA granules when compared to CS and RL (MC<RL<CS). The male species showed more RNA granules in the CB and CBL in comparison to the females. The Table 2 compares the efficiencies ofvaried fixatives in stabilizing the RNA content in the brains of male and female Duttaphrynus melanostictus. The tissues which were fixed with Methacarn showed more number of RNA granules when compared to CS and RL (MC<CS<RL). Similarly to the channastraitathe males of Duttaphrynus showed more RNA granules. The Table 3 compares the efficiencies ofvaried fixatives in stabilizing the RNA content in the brains of male and female Hemidactylus frenatus. The tissues which were fixed with RNA later showed more number of RNA granules when compared to CS and RL (MC<RL<CS). The CNS tissue of Hemidactylus males showed more RNA granules in the CB and CBL. The Table 4 compares the efficiencies ofvaried fixatives in stabilizing the RNA content in the brains of male and female Gallus gallusdomestiucs. The tissues which were fixed with RNA later showed more number of RNA granules when compared to MC and CS (MC<CS<RL). Similarly, the CNS tissue of gallus gallusmales showed more RNA granules in the CB and CBL. The Table 5 compares the efficiencies of varied fixatives in stabilizing the RNA content in the brains of male and female Rattus norvegicus. The tissues which were fixed with RNA later showed more number of RNA granules when compared to MC and CS (MC<CS<RL). The CNS tissue of Rattus norvegicus males showed more RNA granules in the CB and CBL.The cerebral and cerebellar tissues stained with methyl pyronin helped in quantifying the RNA content in brain sections of all vertebrates when fixed in Carnoy’s solution. RNA granules in the cerebrum and cerebellum of where found to be more in Cerebrum and cerebellum of male in comparison to the female. Figures 1-3 shows the photomicrographs of tissue staining with RNA granules. The Figure 4 shows the total RNA in cerebrum and cerebellum of five vertebrate species. The amounts of RNA in male weresignificantly different from female across the five vertebrate species. In the case of toads, avian and rat it was not significant. The ratio cerebrum weight to total brain weight was lowest in Murrel and rat shows that highest ratio. Toads, gecko and avian in between. The same results were observed in male as well as female. Figure 1: Photomicrographs of cerebrum and cerebellum of five different vertebrates fixed in carnoy’s solution (20× magnification; Methyl pyronin staining) Figure 2: Brains fixed with RNA later fixative and stained with Methyl green-pyronin staining of Cerebrum (CB)and cerebellum (CBL) of male and female of five vertebrates
Figure 3: Photomicrographs of brains fixed with methacarn fixative and stained with Methyl green-pyronin staining of different vertebrates
Figure 4: Bar diagram showing total RNA in cerebrum, and cerebellum of five vertebrate species. Mean + SD (n= 6 each). * Males are significantly different from females. (Male: Gecko< Toads < Rat.< Avian <Murrel; Female: Toads < Gecko < Avian < Rat<Murrel) Table 1: Comparative analysis of different tissue fixatives in stabilizing the RNA in male and female Channa striata (Murrel) tissues- Pieces
Table 2: Comparative analysis of different tissue fixatives in stabilizing the RNA in male and female Duttaphrynus melanostictus (Toads)- Amphibians
Table 3: Comparative analysis of different tissue fixatives in stabilizing the RNA in male and female Hemidactylus frenatus (Gecko)-Reptiles
Table 4: Comparative analysis of different tissue fixatives in stabilizing the RNA in male and female Gallus gallusdomesticus--Avians
Table 5: Comparative analysis of different tissue fixatives in stabilizing the RNA in male and female Rattus norvegicus-(Wistar Rats)- Mammals
DISCUSSION Fixation is a process where the structural integrity of cell is preserved from deteriorating. The biological material is fixed using varied fixative that helps by enhancing the stability and mechanical strength of the tissues by terminating the any ongoing biochemical reactions. The main objective of fixatives is to preserve the cellular components (i.e. Proteins, nucleic acids, etc). Many researchers and histopathologists have developed techniques and modified staining procedures to preserve the structural integrity of a specimen for studying and analyze microscopically. Biggest challenge presented to any histologists is to preserve the cell structure from deteriorating immediately when separated from the organs or tissues by using adequate fixation. "Artifacts" are the most common changes that can be observed in the structure of cells and tissues as a result from tissue deterioration and it’s the role of histopathologists in minimizing these so called artifacts and help in distinguishing the intact cells. Another challenge presented here is to make the tissues to withstand the harsh laboratory staining procedures without causing any structural change in a cell and suitable for microscopic examination. Over the years new techniques and chemicals were introduced for the fixation of cells and tissue. It is mandatory for one to know the types of fixative available and choosing an appropriate fixative for a particular purpose or a particular organ. The aim of the current study is tosee the effect of the following fixatives namely Carnoy's fluid, RNA later and Methacarn solution on brain tissues and to observe the optimum result (i.e. RNA quantification)after treating with a particular fixative in Methyl pyronin stained sections. The evolutionary process happening or happened in vertebrates can be elucidated based on the RNA content in their brain tissues. Hence, we choose above mentioned three fixatives for stabilizing and quantifying the RNA in the paraffin embedded brain sections. Observations made from histopathological analysis and RNA quantification experiments clearly suggests that the methacarn is ideal fixative to be employed for fixing and identifying the cellular contents in the brain sections of all the five vertebrates. Whereas, other fixative such as Carnoy’s and RNA later solution did not present ideal condition for stabilizing and identifying the RNA granules in the brain sections of different vertebrates. The methyl pyronin staining has helped in quantifying the RNA in both male and female species of all five vertebrates and it was observed that male brain sections were showing more RNA granules when compared to the female species of all vertebrates (figure 1-3).The total RNA in these five vertebrates was quantified, and it is found that the total RNA content in all these vertebrates differed significantly among these vertebrates and also between genders (figure 4).
CONCLUSIONS In general, decision on the use of RNA preservatives is based on availability of required equipment, expenses, ease of work, handling and preservation periods. If freezing facilities are available and sample collection is centralized, flash freezing as a suitable method for tissue RNA stabilization is preferred. Otherwise, the use of chemical preservatives such as sulfate solution or TRIzol may be advisable. In this circumstance, if preserved tissue is intended for both molecular and histopathological studies, the commercial compounds such as RNAlater, Allprotect and PAXgene would be recommended However, apart from preservation methods, other parameters such as timing of tissue collection and preservation, use of different fixatives, RNA extraction procedures, tissue quantity and checking methods for RNA quantity and quality would also directly or indirectly influence RNA integrity and gene expression.
REFERENCES
Policy for Articles with Open Access: Authors who publish with MedPulse International Journal of Pediatrics (Print ISSN: 2579-0897) (Online ISSN: 2636-4662) agree to the following terms: Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal. Authors are permitted and encouraged to post links to their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home