|
Table of Content Volume 5 Issue 3 - March 2018
Histogenesis of foetal thymus: A microstructural study in human
J K Sarkar1, B C Dutta2*
1Associate Professor, 2Professor, Department of Anatomy, Silchar Medical College, P.O.: Ghungoor, Silchar-14, Cachar -788014, Assam, INDIA. Email: jksarkar31@gmail.com, drbijondutta@gmail.com
Abstract Aim and Objectives: Aim of the study was to find the appearance of lobulation, lymphocyte cortico-medullary differentiation and Hassall’s corpuscles in thymus at different age groups of human foetus. Our objective was to observe the various histological changes that occurred in foetal thymus with the development of foetus at different gestational periods. Materials and Methods: Sample from 90 human foetuses were taken ranging from 9th -38th week of gestation. After tissue processing and fixation, sections were stained with Haematoxylin and Eosin and Leishman’s stains. The slides were examined under optical microscope. Results and Observation: Appearance of lobulation was observed at 9th week and completed by 12th week of gestation. The differentiation of cortex and medulla started at 9th week and completed between 12th and 14th weeks. The Hassall’s Corpuscles were observed at 14th week and its number and size increased during 15th -24th weeks. Monocytes and Macrophages were observed at 12th week. Conclusion: The study revealed significant microstructural changes of foetal thymus such as lobulation, differentiation of cortex and medulla. The appearance of Hassall’s Corpuscles occurred at 14 weeks and their number and sizes increased between 15-24 weeks of gestation. Key Words: Foetal thymus, gestation, lobulation, cortex and medulla, Hassall’s Corpuscles.
INTRODUCTION The thymus is a soft bi-lobed primary lymphoid organ, producing immunocompetent T-cell and responsible for cell mediated immunity of the human body. It is surrounded by a mesoderm derived collagenous connective tissue capsule, penetrate the epithelial thymus along with blood vessels during foetal development to form discrete lobules. Each lobule can be divided histologically into an epithelial cell-rich medulla surrounded by lymphocyte-rich subcapsular and inner cortex1. The lobules are not completely separate units as the medulla constitutes a central core to each lobe and sends prolongations into each lobule2. Hassall’s corpuscles 30-100μm in diameter are exclusively found in the medulla and function as the graveyard of thymocyte or they represent remnants of medullary duct epithelium3. Macrophages are found throughout the thymus and conspicuous in involuted thymus. Myoid cells are relatively rare, situated mainly in medulla and at the corticomedullary junction4. The right and left primitive thymic rudiments formed at 4th week of gestation, moving caudally to fuse at the midline at the beginning of 8th week of gestation. Later on, blood-borne T-cells precursors from the bone marrow begin to colonize the epithelial thymic rudiment at 8th week. In the foetus at 10th week, mesodermal derived fibrous connective tissue surrounding vessels begins to invaginate the thymic rudiment effecting thymic epithelial proliferation and thymic lobulation1. The thymus is lobulated by 12th week and cortico-medullary differentiation is completed at 14th week5.The non-lymphoid components of the thymic microenvironment (epithelium, fibroblast, macrophages) play critical roles in normal thymic development. The thymus contains stem cells for mast cell differentiation and eosinophilopoeisis requires the presence of the thymus.
MATERIALS AND METHODS The study was carried out in the Department of Anatomy on 90 (48 male and 42 female) human foetuses ranging from 9-38 weeks of gestation. The foetuses were obtained from the Department of Obstetrics and Gynaecology, Silchar Medical College and Hospital, Silchar following spontaneous abortion, elective termination and stillbirth. The caseswere selected where no gross congenital anomalies, marked oedema, obvious maceration or microscopic signs of autolysis were seen. The foetuses were procured one to ten hours after delivery and preserved in 10% formalin solution. Most of the thymuses were examined within a few hours of delivery not going beyond 24 hours.The thymus was taken out from mediastinum by disarticulating sternoclavicular joint and cutting the costal cartilages. The tissues of average thickness about 5mm taken from upper, middle and lower parts of each lobe of all thymuses and fixed in 10% formalin. Then tissue dehydrated with 50%, 70%, 90% and absolute alcohol and cleared with xylene and chloroform. These were then embedded in paraffin. Serial sections of 5-10 microns thickness were cut with rotary microtome. The prepared sections were stained with Haematoxylin and Eosin and Leishman’s stain. The stained sections were then examined under optical binocular and trinocular microscope. The sections showing maximum clarity were chosen for photomicrography.All findings of this present study were then compared with findings of different researcher and their literature. RESULTS AND OBSERVATIONS
Table 1:
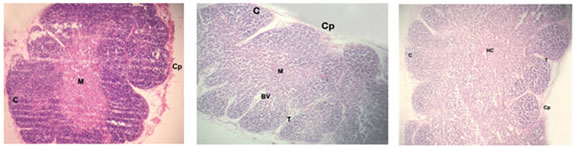
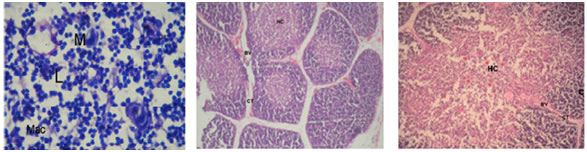
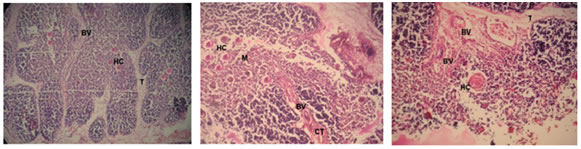
Figure 1 Figure 2 Figure 3 Figure 4 Figure 5 Figure 6 Figure 7: Figure 8: Figure 9: Figure 1: Photomicrograph of foetal thymus at 9 weeks showing Capsule (Cp), appearance of lobulation, development of cortex (C) and medulla(M). (Haematoxylin and Eosin, 10X); Figure 2: Photomicrograph of Foetal Thymus at 12 weeks showing lobulation, capsule (Cp), Cortex (C) and medulla (M), Trabeculae (T) containing Blood Vessels (BV). (H and E, 10X); Figure 3: Photomicrograph of human foetal thymus at 14 weeks of gestation. Capsule (Cp), Trabeculae (T), Cortex (C), Hassall’s Corpuscle (HC) are shown. (H and E);Figure 4: Oil-immersion Photomicrograph of foetal thymus stained with Leishman’s stain showing Lymphocytes (L) and Monocyte (M) and Macrophage (Mac); Figure 5: Photomicrograph of Foetal Thymus at 17 weeks of gestation showing connective tissue trabeculae (CT), Blood vessels (BV), Cortex (C) and Hassall’s corpuscles (HC). (H andE, 10X); Figure 6: Photomicrograph of a lobule of human foetal thymus at 20 weeks showing interlobular connective tissue (CT), Blood vessels (BV), Cortex (C) and Hassall’s corpuscles (HC) in medulla. (H and E, 80X); Figure 7: Photomicrograph of Human Foetal Thymus at 24 weeks gestation showing Trabeculae (T), Blood vessels (BV), Hassall’s corpuscles (HC) in medulla. (H and E, 40X); Figure 8: Photomicrograph of Human Foetal Thymus at 32 weeks of gestation showing Cortex (C), Hassall’s corpuscles (HC) in Medulla (M), Blood vessels (BV) in interlobular connective tissue (CT). (Haematoxylin and Eosin, 40X); Figure 9: Photomicrograph of Human Foetal Thymus at 38weeks showing Hassall’s corpuscles (HC), Blood Vessels (BV) in Trabeculae (T). (H andE, 160X) The foetuses of 9-38 weeks of gestation were divided into seven groups (Table-I) and observations were noted as under: - Group I: 9-11 Weeks: The gland had a delicate capsule containing blood spaces. Lobulation was evident at this stage. The development of cortex and medulla had started. The medulla was relatively pale and cortex darkly stained. The lymphocytes were more in the cortex. Trabeculae containing blood vessels were also observed. (Fig. 1and 2) Group II: 12-14 Weeks: The lobulation of the thymus continued and each thymic lobule had well defined cortex and medulla. The cortex was highly cellular whereas medulla was paler and loosely packed containing fewer lymphocytes. Connective tissue extended from capsule into depth of the gland forming interlobular septa containing blood vessels. Macrophages and Monocytes were also observed (Fig.4). Hassall’s corpuscles appeared at this stage. (Fig. 3) Group III: 15-17 Weeks: The lobulations of the gland were still continuing and interlobular septa containing blood vessels were prominent. The relatively paler medulla ramified into each lobule. The darkly stained cortex packed with lymphocytes. The numbers and size of Hassall’s corpuscles were increasing in all sections. (Fig. 5) Group IV: 18-20 Weeks: Trabeculae invading the gland containing blood vessels were more prominent. The cortex was predominantly populated with lymphocytes. Hassall’s corpuscles increased in size and numbers in all sections. (Fig. 6) Group V: 21-24 Weeks: The thymus was highly lobulated. In random sections some of the lobules appeared to be completely separated from adjacent lobules. Hassall’s corpuscles increased further in size and their numbers. (Fig. 7) Group VI: 25-32 Weeks: Lobular architecture, trabecular framework and blood vessels were more prominent. Thymic tissue of each lobule was continuous in the more central part of each lobe with that of other lobule. The cortex was heavily infiltrated with lymphocytes. Blood vessels supported by connective tissue seen in the substance of lobules. (Fig 8) Group VII: 33-38 Weeks: The vascularity of the thymus was markedly increased which were observed in interlobular connective tissue as well as within the substances of lobules. Hassall’s corpuscles were numerous in medulla showing increase in size as well as maturity (Fig 9).
DISCUSSION The present study revealed several points having marked importance in practical life. Hence these have been considered worthy of discussion in conformity with the findings of other researcher to draw definitive conclusion in respect of microstructural changes of thymus among different age groups of developing human foetuses. Appearance of lobulation: Haar and Ghali et al reported appearance of lobulation by 12 weeks5 and 10 weeks of gestation6 respectively. In the present study lobulation began to appear at 9 weeks (Fig. 1) and well distinct lobules were observed from 12 week onwards (Fig. 2). Cortex and medulla: Hayward reported that cortex and medulla were distinguishable around 12 weeks7. Haar reported that at 14 weeks, cortex and medulla could be distinguished and by 18 weeks, a matured looking cortex and medulla were observed5. Ghaliet alreported that cortico-medullary differentiation was distinct at 11 weeks6.On the contrary, the present study was comparable to the observations ofVon Gaudecker B8 and Ajitaet al12 that between 12th and 14th gestational week, cortical and medullary differentiation was completed.Lobach D F and Haynes B Fobserved histologic distinction between cortical and medullary zones at 14 weeks1. Larsen W J reported that by 12 weeks, each thymic lobules had a well-defined cortex and medulla9. In the present study, cortico-medullary differentiation began to appear at 9 weeks (Fig. 1) and well differentiated cortex and medulla were observed between 12 and 14 weeks of gestation (Fig. 2 and Fig. 3) Blood vessels: Haar5reported intrathymic vessels composed of endothelial cells and basal lamina with epithelial cells and their extraneous coat at 9 weeks of gestation. By 12-14 weeks of gestation, vessels were found within the lobules of the thymus. Ghaliet alreported that the thymus was vascular since 10th week6. Lobach D F and Haynes B Freported that at 10th week of foetus, blood vessel had begun to invaginate the thymic rudiment1.Muresian H reported that blood vessel present at 12 weeks.In the present study, blood vessels were observed in connective tissue capsule and trabeculae from 12 weeks onwards (Fig. 2). Lymphocytes: Carr et al (1975)reported that the growth rate of lymphocytes in 7 -20 weeks of human foetuses were linear when plotted logarithmically11. Sahana S Nreported that lymphocytes appeared into the gland at 10 weeks. However, the present study revealed the presence of lymphocytes from 9 weeks of gestation (Fig. 1). The present observation is comparable with Haar5, Von Gaudecker B8, Hamilton Boyd and Mossman10 and Ajitaet al12. Epithelium: Shier K J reported thatprominent epithelium lined tubules were demonstrable in the mesenchymal interstitium of foetal thymus up to the 4th lunar month13. Haywardobserved that epithelial components of the thymus was recognizable at 10 week. Haar cited that at 9 weeks of gestation some epithelial cells at outer surface of the thymus were columnar. Haynes B Fobserved that thymic epithelial component first appeared at 8-9 weeks14. Kendal M D reported that medullary epithelial cells fully formed by 17 gestational week15. However, in the present study, epithelial cells were observed from 14 weeks onwards of gestation. Macrophages: Haar observed occasional macrophages within the developing thymus after 12 weeks gestation5. Muresian H reported that macrophages and interdigitating cells were first seen at 14 weeks4. Whereas, the present study revealed the presence of macrophages from 14 weeks onwards of gestation. (Fig. 4) Hassall’s corpuscles: Ghaliet al observed that tiny Hassall’s corpuscles were first formed at 11 weeks6. Lobach D F and Haynes B Freported thatHassall’s corpuscles formed in the medulla between 15 and 16 weeks1. Haynes B Freported that Hassall’s corpuscles were presentat 15 weeks14. Bodey Bobserved that between the 13th to 16th weeks, the first Hassall's bodies were developed. Presence of Hassall's Corpuscles was observed from 14th week by Prabavathy G and was found in all sections from 15th week onwards16. Bodey B and Kaiser H E detected the development of the first HB during the second part of the third intrauterine lunar month in human foetuses. The greatest developmental progression and main cell-tissue organization of the Hassall’s corpuscles was observed between 6 and 10 lunar month17. However, in the present study, Hassall’s corpuscle was first observed at 14 weeks of gestation which showed increase in size and numbers during 15-24 weeks (Fig. 3).
CONCLUSION The thymus is bilobed gland that changes in microstructure with gestational age of the foetus. Each lobe is composed of thousands of lobules. The lobulation starts at 9th week. The cortico-medullary differentiation starts at 9 weeks and completed by 14 weeks. The lobules are not completely separate unit as medulla constitute a central core to each lobe and send prolongations into each lobule. A capsule encloses each lobe and extension from it (inter lobular septa) delineate the lobules. The cortex is darkly stained containing densely packed lymphocytes. Lymphocytes were observed at 9 weeks of gestation. The medulla stains lightly with prominent epithelial reticular cells. Hassall’s corpuscles are exclusively present in medulla.The first appearance of the Hassall’s corpuscles was recorded at 14 weeks of gestation which show increase in size and numbers during 15-24 weeks. The blood vessels present in capsule and interlobular septa of the thymus are noted since 12 weeks onwards. Macrophages are also observed from 12 weeks onwards.There was no significant sex difference between male and female foetus in regards to their microstructures of the thymus. Hassall’s corpuscles are unique, multicellular non-lymphatic components of cellular micro-environment of thymic medulla. The thymus is critically required for the production of the vast majority of T cells.The lymphopoiesis is a function of thymic microenvironment which is useful andcan be obtained from human foetal lymphoid organs for using in transplantation in immune deficiency diseases and other clinical conditions.
REFERENCE
|
|
 Home
Home