Official Journals By StatPerson Publication
|
Table of Content - Volume 10 Issue 3 - June 2019
Study of analyte stability time of serum electrolytes in a tertiary care public hospital
Dipti Tiwari1*, Pramod Ingale2, Shubhangi Wankhade3, Minal Pore4
1Assistant Professor, Department of Biochemistry, Grant Government Medical College and Sir JJ Group of Hospitals, Mumbai, INDIA. 2Professor and HOD, 3Speciality Medical Officer, 4Speciality Medical Officer, Department of Biochemistry, Lokmanya Tilak Municipal Medical College, Sion, Mumbai, INDIA. Email: tiwari.dipti02@gmail.com
Abstract Background: The collected and shipped blood samples are exposed to various extra-analytical factors prior to analysis. At times there is a delay in sample analysis after centrifugation due to a large sample load, breakdown of the analyzer and the nonavailability of a backup system. With this back ground we took up this study to see the effect of time on the assayed values of serum electrolytes. Material and Methods: We analyzed 152 samples for serum electrolytes at different time intervals between centrifugation and sample analysis on automated analyzer. The samples were received from different wards within one hour after sample collection. The samples were immediately analyzed after centrifugation and values recorded. The samples were kept in the lab uncovered as per the usual practice, with controlled environmental temperature conditions (24 ± 2˚C) and were estimated twice after a gap of 2 hours each. i.e. at 3 hours and 5 hours post sample collection. All the samples were analyzed on the same instrument using Beckman AU680 analyzer on ISE mode. Results: Results compared using repeated measure ANOVA which showed statistical difference P < 0.05 between 1st and 2nd, and 2nd and 3rd measurement. There was statistically significant rise in values of Na and K over a period of time.Conclusion: Evaporation of sample could be the major cause leading to sample concentration resulting in high values. Simple measures like early analysis of samples or covering the samples properly will prevent such erroneous results. Key Word: Sodium and Potassium, Electrolytes
INTRODUCTION Laboratory testing is a complex process which involves a series of interrelated steps and each step is prone to error. According to the draft of the ISO technical report 22367, laboratory error is defined as “a defect occurring at any part of the laboratory cycle, from ordering tests to reporting results and appropriately interpreting and reacting to these” . Laboratory error leads not only to unnecessary delays and additional costs by necessitating obligatory repeat samples but also imparts unnecessary pain to the patient. Laboratory testing is divided into three phases: preanalytical, analytical and post analytical. Most errors occur in the preanalytical phase (46-68.2%) and the post analytical phase (18.5- 47%) but still a large fraction (4-32%) can be attributed to the intra analytical phase of the testing process . Laboratory errors in the analytical phase have significantly decreased in recent times due to automation and technological advancements. The transition phase between the preanalytical and the intra analytical phase is also considered to be an important error prone area. Analyte stability time described by World Health Organization (WHO) and Clinical and laboratory Standard Institute (CLSI) is often difficult to apply in the clinical settings as the time taken to transport samples from collection centre to laboratory and the time interval between centrifugation and processing are usually laboratory dependent variables. Biochemical investigations routinely ordered by the clinicians are primarily blood glucose, liver function tests, kidney function tests and serum electrolytes. Electrolyte analysis includes sodium, potassium and chloride. Na+ and K+ form major cations of body fluids. Na+ is the most abundant extracellular cation maintaining the osmolarity while K+ is the most abundant intracellular cation maintaining the resting membrane potential and thus associated with conductivity of electrical impulse. Accurate estimation of serum electrolytes has gained importance in diagnosis of etiology of various diseases as it is used to calculate Anion Gap. Electrolyte abnormalities can precipitate life threatening events by derangements in its metabolic process or as a consequence of an underlying disease. Hyponatremia may be associated with sodium losses due to vomiting or diarrhea, diuretics abuse, salt losing nephropathy and osmotic diuresis, metabolic acidosis, adrenocortical insufficiency, dilution type hyponatremia may be due to edema, cardiac failure, hepatic failure and hypothyroidism. Hypernatremia is associated with conditions with water loss in excess of salt through profuse sweating, severe vomiting or diarrhea, diabetes insipidus or diabetic ketoacidosis, hyperaldosteronism, Cushing’s syndrome, inadequate water intake because of coma or hypothalamic disease, dehydration or excessive saline therapy. Potassium plays a role in nerve conduction, muscle function and helps maintain acid-base balance and osmotic pressure. Measurement of serum potassium is used for evaluation of electrolyte imbalance, cardiac arrhythmia, muscular weakness, hepatic encephalopathy, monitoring of diabetic ketoacidosis and intravenous fluid replacement therapy. Electrolyte abnormalities are one of the common reversible causes of morbidity and mortality in Intensive Care Unit (ICU) patients . Analysis of these electrolytes dictate management protocol as well as outcome. There lies enough controversy regarding how quickly a serum sample has to be processed for them after collection. In a tertiary care set up, bulk samples are routinely received by the laboratory. Processing these samples therefore require sufficiently longer time periods and hence there are chances that the sample may deteriorate over a period of time giving inaccurate results. The development of blood collection tubes that contain gel and form a barrier after centrifugation has markedly improved serum analyte stability in the primary tube , , . There are few published studies on analyte stability in laboratories where there is a delay before centrifugation. Other studies have looked at the stability of various analytes in whole blood, however, the conditions to which the specimens were exposed have usually been within manufacturer's defined limits , , , . The Asia Pacific region has unique challenges arising from its vast geography and climatic conditions. Regional and remote health-care workers often do not have access to a centrifuge. This results in a delay of centrifugation and exposure of the specimen to variable temperatures and times during transport before being centrifuged .In majority of tertiary care hospitals, sample collection is done in the wards and the outpatient department by trained laboratory phlebotomists and are then transported to laboratory. The samples are then centrifuged and analysis is carried out using either standalone ISE analyzer or on fully automated clinical chemistry analyzer based on ISE method. Many a times there is a delay in sample analysis after centrifugation due to a large sample load, breakdown of the analyzer and the non-availability of a backup system. Hence variations in these electrolytes which do not correspond to the clinical state of the patient are commonly observed and repeat samples are required to be processed so as to get the reliable result. We therefore sought to evaluate the time lag between centrifugation and sample analysis which affect the stability of electrolytes in serum and find out the optimum time in which the samples need to be analyzed. AIMS AND OBJECTIVES
MATERIALS AND METHODS The study was conducted at Lokmanya Tilak Municipal Medical College, Sion, Mumbai. 152 samples collected from different wards were included in the study. Haemolysed, icteric and lipemic samples were excluded from the study. All samples were analyzed on the same instrument i.e. Beckman Coulter AU680 on ISE mode. Serum electrolyte values estimated immediately after centrifugation (within 1hour of sample collection) and then twice, after a gap of 2 hours each (3 and 5 hours post sample collection). Samples were kept uncovered in the lab as per the usual practice of the lab. Graph Pad prism was used for statistical analysis. Analysis of variance and paired-t test done on the values of serum electrolytes at three different time intervals.
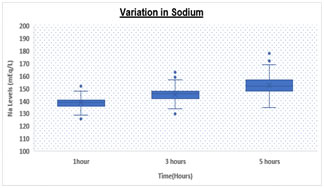
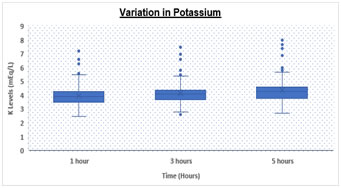
RESULTS Table.1 shows a significantly (p < 0.05) elevated serum sodium levels at 5hrs (152.67 ± 7.1 meq/L) as compared to 3hrs (145.27 ± 5.3 meq/L) and 1hr (139.08 ± 4.1 meq/L) respectively. We also observed significantly (p < 0.05) increased levels of serum potassium levels at 5hrs (4.36 ± 0.8 meq/L) as compared to 3hrs (4.16 ± 0.8 meq/L) and 1hr (3.98 ± 0.8 meq/L) respectively. Table 1: Comparison of Sodium and Potassium at different time intervals
*SD – Standard Deviation
Box and Whisker Plot: Showing variation in Sodium and Potassium levels DISCUSSION There was statistically significant rise in Na and K values (p value <0.05) over a period of time. Both analytes showed a significant difference at 3 hours and 5 hours with a p value of < 0.05 for both. However, K values showed higher rise when analyzed after 5 hours of collection as compared to 3 hours while Na values increased with a higher rate during initial 3 hours as compared to 3-5 hours. Various studies have been conducted in the past to demonstrate the stability of many analytes. Donnelly et al, 1995 who investigated the stability of 25 analytes showed that Na and K remain stable for 24 at room temperature 4˚C to -20˚C . Boyanton et al, 2002, found that Na and K remain stable up to 56 hours . However, study by Tanner et al, 2008, on 35 analytes showed that stability of K is altered within 24 hours but sodium remains stable up to 24 hours. In all studies on analyte stability strict temperature maintenance is followed . This gross difference between other stability studies and our study could be due to improper temperature maintenance and open sample tubes kept in laboratory leading to significant evaporation of samples. We are continuing the study further under strict temperature -controlled environment and capping all the tubes. CONCLUSION Evaporation of samples could be the major cause leading to concentration of samples resulting in high values. To conclude we suggest that the samples for measurement of serum electrolytes should be analyzed as soon as they are received in the laboratory preferably within 1-2 hour. In the event of any delay, sample cups should be properly covered and stored under proper environmental conditions to avoid erroneous results.
REFERENCES
|
 Home
Home