Official Journals By StatPerson Publication
|
Table of Content - Volume 11 Issue 2 - August 2019
Study the correlation of iodine nutrition and autoimmunity among thyroid disorder children in Tamil Nadu
Suresh Pichandi1, Jeyakumar M2*, Janakiraman P 3, Ramadevi K4
1Associate Professor, Department of Biochemistry, Sri Venkateswaraa Medical College and Research Center, Puducherry, INDIA. 2Assistant Professor, Department of Biochemistry, Velammal Medical College Hospital and Research Institute, Madurai, Tamil Nadu, INDIA. 3Tutor in Statistics, Department of Community Medicine, PES Institute of Medical Sciences and Research, Kuppam, Andhra Pradesh, INDIA. 4Professor and HOD,Institute of Biochemistry, Madras Medical College and Govt Rajiv Gandhi General Hospital, Chennai, Tamil Nadu, INDIA. Email: suresh.chanth82@gmail.com
Abstract Objective: Aim of the present study is to analyse the nutritional status of iodine among paediatric patient by measuring urinary excretion and correlate with thyroid hormone and auto antibodies Materials and Methods: Two hundred paediatric patient with thyroid disorder and two hundred age-matched normal children were included in study. Urinary Iodine excretion and serum TSH, fT4, fT3, AMA and ATG were estimated were estimated for case and control groups. Results: The mean urinary iodine excretion concentration (UIC) in paediatric patient was 287.60 µg/L and excess urinary iodine excretion was found in 51%. There were elevated serum AMA levels in 68% paediatric patient and that positive correlated with excess iodine. Conclusion: In this study we found that excess urinary iodine excretion among all patients. Autoimmune thyroid it is was seen 68% of patients. Key Word: Urinary iodine excretion (UIE), thyroid stimulating hormone (TSH), free thyroxine (fT4), free tri-iodothyronine (fT3), anti-microsomal antibody (AMA), anti-thyroglobulin antibody (ATA), pregnancy, iodine excess
INTRODUCTION Iodine is a trace element that is essential for the formation of thyroid hormones that required for cell differentiation, and the development of the central nervous system1,2. It is important for fetal and infant neurological growth3. So, iodine is crucial for the growth and development4. The recommended daily intake of Iodine for different age groups is given in Table 15. Iodine deficiency may result in a spectrum of disorder called iodine deficiency disorders (IDD), including stunted physical growth, squint, deafness, stillbirths, abortion, impaired mental abilities, neonatal cretinism and hypothyroidism.6 Universal salt iodination programme implemented to eradicate the IDD. Community based assessment the impact of USI program, urinary iodine measurement is a good marker of dietary intake of iodine recommended by WHO.7 After universal salt iodization, several reports has been published that severe iodine deficiency has been eliminated however iodine excess has steadily increased the number of countries in over the past decade8-10. Fetuses and newborn children are particularly vulnerable to iodine excess, since their immature thyroid gland is unable to escape from the Wolff-Chaikoff effect11-13, which may lead to long-te rm thyroid dysfunction14 Despite the intake of iodized salt, the increasing occurrence of thyroid disorders has prompted us to investigate the iodine status among these patients and to correlate with the thyroid function.
Table 1: WHO recommendation for daily dietary intake of iodine
SUBJECTS AND METHODS The 200 paediatric samples from ages between 6 to 12 years were collected from Institute of Child Health, Madras Medical College, Chennai. A total of 200 children with thyroid dysfunction such as hypo and hyper thyroid and goitrous enlargement of thyroid gland, were included in this study and their urinary iodine excretion, thyroid hormone status, thyroid auto antibodies were analysed. Two hundred non-goitrous healthy age and sex matched thyroid volunteers were the control group. All the children samples were estimated the thyroid hormones (TSH, fT4 and fT3 by ELISA method) and urinary iodine (15) were measured. The antibody titres anti-microsomal antibodies (AMA) and anti-thyroglobulin antibodies (ATG) (by ELISA method) were also measured in all paediatric samples. Statistical Analysis: The data processing and analysis were done by STATA 13 version software. The quantitative data of case and control were presented as mean and standard deviation and comparison of means was done by student t-test. The categorical data analysed by Chi-square (χ2). The nonparametric data were expressed as median and inter quarter range (IQR) and comparisons among the groups were done by using Kruskal-Wallis test. The Pearson’s correlation test was used to find associations between analytes.
RESULTS The mean age of paediatric patient was 9.22 ± 2.04 years while the controls children had 10.36 ± 0.84 years. The mean urinary iodine excretion patients group was 287.60 ± 98.34 µg/L while the controls had 158.82±76.76 µg/L (table 2). There was significant difference in the UIE between the paediatric patient and control (p value<0.001). The result shows that excess urinary iodine excretion among the thyroid disorder paediatric patients.
Table 2: thyroid profile status for care and control
Value are expressed mean±SD; statistical significance by t-test; *P<0.05) **p<0.01 significantly different between two group;
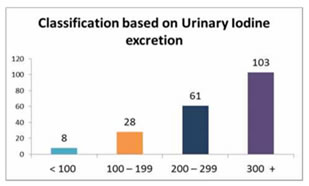
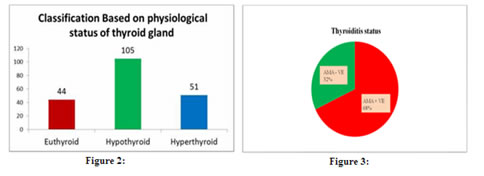
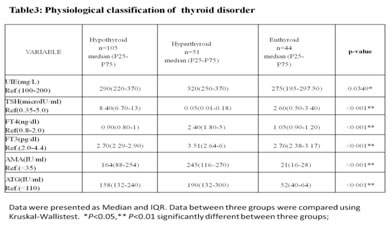
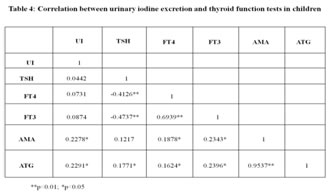
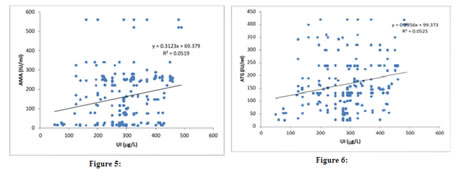
Based on the results obtained, the patients were classified into 2 types: Type I: Urinary iodine excretion: Based on the urinary iodine level, the 200 paediatric patients were classified into four groups [Fig. 1]. Using the World Health Organization (WHO) criteria, iodine nutrition, as assessed by the mean urinary iodine excretion of paediatric patients, is described in [Fig. 1]. Only 4% of the children patients had iodine deficiency, while 82% had more than adequate iodine nutrition and among that 51 % had very high excretion of iodine (>300µg/L), while 14% had normal iodine status. Figure 1: classification based on urinary Iodine excretion Type II: Physiological classification: Based on the thyroid hormone profile, and antibody titters, the 200 paediatric patients were classified into three groups as hypothyroid (n=105), hyperthyroid (n=51) and euthyroid (normal thyroid) subjects (n=44) [Fig.2]. The anti-microsomal antibody was positive in 68% paediatric patient’s subjects. (Fig. 3) The comparison was done among hypothyroid, hyperthyroid and euthyroid paediatric patient’s subjects, median value of urinary iodine were 290(220-370), 320(250-370) and 275(195-297.50) respectively and there were significant difference (p value<0.05) in the median urinary iodine excretion across these groups (Table 3 and Fig. 4). Pearson’s correlation was used to find the correlation and association between analysts. There was a significant correlation between UI and AMA (r= 0.2278) (p value 0.01) in paediatric patient’s subjects. Also, a significant correlation between UI and ATG (r= 0.2291) (p value 0.01) was found in those subjects. (Table 4 and Fig. 5, 6). Figure 2: Classification based on physiological status of thyroid gland; Figure 3: thyroiditis status
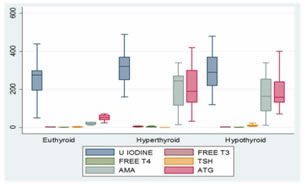
Figure 4: comparison of thyroid disorder based on physiological status of thyroid gland Data were presented as median inter quarter range (IQR) data between three groups were compared using kruskal-wallis test
Figure 5: UI vs. AMA; Figure 6: UI vs. ATG
OBSERVATION Iodine is an essential micronutrient throughout life which participates in the normal mental and physical development and maintenance of homeostasis in humans. Deficient or excessive iodine intake may affect thyroid gland size and functions.(16) Iodine deficiency has been endemic in India for a long time. Iodine sufficiency has been achieved in many parts of the country after Universal Salt Iodization (USI) in India. In our study showed iodine excesses (above 200µg/L ) in 82%of the subjects among that 51 % had very high excretion of iodine (>300µg/L), while 14% had normal iodine status (MUI 150-250 µg/L).Only a small percentage (4%) of the study group had iodine deficiency (less than 100µg/L).The study group are classified based on thyroid hormone as hypothyroid (n=105), hyperthyroid (n=51) and euthyroid (normal thyroid) subjects (n=44) and there median value of urinary iodine was 290(220-370), 320(250-370) and 275(195-297.50). These reports indicating that excess urinary iodine excretion are hall mark for paediatric patient subject Thyroiditis was observed in 68% of patients in this group. The excess iodine may be the reason for triggering autoimmunity in this study group. The correlation was done to assess the strength and relationships between the urinary iodine and thyroid hormones and auto-antibodies among paediatric group. The results of the present study showed good agreement among the different thyroid hormones with excess urinary iodine excretion. Urinary iodine was positively correlated with anti-microsomal antibodyAMA (r= 0.2278) (p value 0.01), and anti-thyroglobulin antibodyATG (r= 0.2291) (p value 0.01). The higher MUI in paediatric subjects may be due to an improvement in the implementation of USI, over time. Limitations of our study is small hospital based study, Larger sample size community-based studies are required in populations, to establish the reference ranges for urinary iodine concentration in pregnancy. Also in the hospital settings could not identify the Iodine status of the populations with respect to the dietary habits. CONCLUSION The chances of iodine deficiency are much less in coastal areas like Tamil Nadu because of adequate amount of iodine is available in the coastal regions of our country. Universal salt iodination program has also eliminated the iodine deficiency as reported by many studies. Iodine excess is the hall mark that is observed among our patients in this study. The study comprised of 200 paediatric patient. The study group had a mean UI excretion of 287.60 µg/l. This shows majority of the pregnant women had increased urinary iodine excretion and suggested excess iodine intake. These group women had 68 % AMA positive. Also, our study report showed positive correlation of urinary iodine with auto antibodies AMA and ATG. Excessive iodine intake may trigger thyroid autoimmunity. Risk–benefit analysis clearly is in favour of iodine supplementation, but at the same time speaks for a careful dosing, avoiding median intakes above the upper recommended level. This small hospital-based study for urinary iodine concentration in paediatric patient may not be representative of community iodine nutrition and similar large number of sample required for community settings to find the iodine nutrition in children. From this study we recommended that young patients who present with thyroid dysfunction should be evaluated for auto immune thyroiditis and thyroid function and urinary iodine. The time of diagnosis need to be followed up with periodic assessment of thyroid function, which will aid in early detection of thyroid dysfunction to prevent adverse effects on growth and development.
REFERENCES
|
 Home
Home