|
Table of Content - Volume 18 Issue 3 - June 2021
Study of changes in erythrocyte lipid peroxidation and antioxidant potential during storage of blood
Chhaya Keny1, Sucheta Dandekar2*, Jayashree Sharma3
1Technical Supervisor, 3Professor And Head, Department of Transfusion Med., Kem Hospital, Parel, Mumbai, INDIA. 2Professor, Department of Biochemistry, Era University, Era's Lucknow Medical College, Uttar Pradesh, INDIA. {Adjunct Faculty, Manipal Aca of Health Education, INDIA.} Email: chhayakeny.ck@gmail.com, sucheta.dandekar@gmail.com, jayashreesharma@kem.edu
Abstract Background: Oxidative damage is the most important factor causing RBC storage lesion. Free radicals can damage RBC products by lipid and protein oxidation affecting cell quality. The present study is aimed to study the effects of lipid peroxidation and potential role of enzymatic antioxidants in stored blood. Material and methods: This observational study was carried out in healthy blood donors at KEM hospital, Mumbai. Thirty healthy donors, who were fulfilling the inclusion and exclusion criteria, were enrolled in the study. Estimation of hemoglobin, levels of lipid peroxidation and some enzymatic antioxidant activity were carried out in a properly stored blood samples at 40C. Enzyme levels estimation was carried out at every 7 days interval. Results: Malondialdehyde (MDA) levels in the study indicate that lipid peroxidation in red cells has occurred in the preservation period. Throughout storage period, the levels of glutathione peroxidase and catalase declined. Statistically significant negative correlation existed between lipid peroxidation and glutathione peroxidase. Blood grouping of all samples indicate no significant susceptibility to lipid peroxidation when the different blood groups were compared, although some changes were marginally evident in O+ ve group. Conclusion: Red cell storage lesions due to oxidative injury during storage are now the reported fact, confirmed by the findings of the present study. Further large sample studies now will be required investigate the therapeutic role of antioxidants in preventing oxidative damage to red cells during storage. Key words: lipid, antioxidant, catalase, peroxidase, glutathione, enzymes.
INTRODUCTION The history of blood transfusion originated with William Harvey’s discovery of blood circulation in 16281. The earliest known blood transfusions took place in 1665 by Richard Lower.2 Technology making the transfusion of allogeneic blood products feasible includes Karl Landsteiner’s landmark identification of the human blood groups A, B, and O in 1901.3 Decastello and Sturli added the fourth group, AB, in 1902. The life saving potential of blood and its components has been truly known since the last century and hence the importance of blood transfusion. To maintain viability and activity of red cells, blood collected from the donor has to be stored under some physiological conditions until it is transfused to the recipient. The First World War acted as a catalyst for the rapid development of blood banks and transfusion techniques. For storage of blood, the basic physical and chemical conditions are generally optimized that include temperature, pH, the source of energy of red cells and use of anticoagulant. Red Blood Cells (RBCs) are perhaps the most recognizable component of whole blood. The ability to store RBCs and other components for extended periods of time has dramatically expanded the availability and use of transfusion as a life-saving therapy. However, as soon as whole blood is collected from a donor, red blood cells begin to degrade. RBCs experience progressive biochemical and biomechanical changes during storage, collectively called the “storage lesion”, that result in compromised physiological functions. Free radicals are highly reactive molecules generated by biochemical redox reactions that occur as a part of a normal cell metabolism and in the course of free radical mediated diseases such as cancer, diabetes mellitus, cardiovascular and renal diseases.4 Free radicals may cause lipid peroxidation (the level of lipid peroxidation expressed as malondialdehyde) and damage macromolecules and cellular structure of the organism, endothelium and erythrocytes. Plasma Malondialdehyde (MDA) is the breakdown product of the major chain reactions leading to definite oxidation of polyunsaturated fatty acids such as linoleic and linolenic acid and thus serves as a reliable marker of lipid peroxidation.5,6 Free radicals are eliminated from the body by their interaction with enzymic and non enzymic antioxidants such as uric acid, albumin, vitamin E, C, A, glutathione, glutathione peroxidase, superoxide dismutase and catalase.4 There are few reports describing the use of stored blood without any alteration in biochemical factors. In a study, Acid-Citrate-Dextrose was used as preservative in stored blood in 1947.7,8 In other study, Citrate-phosphate-Dextrose and Citrate-phosphate-Dextrose-Adenin were used as blood preservative factor in 1957 and 1960 respectively. In further study, Glucose was added to the final constituent and the CPDA-1 was formed and the useful blood storing time increased to 35 days which with adding of Mannitol to CPDA-1, this time increased to 42 days.9 There are some reports about reducing of red blood cell life span and antioxidant status in the stored blood which shows there are some changes on plasma level of free radicals and antioxidants. Blood is permanently exposed to oxidation stress and therefore, it has a high antioxidant capacity.10 In the stored blood of donors, many factors increasing the demands on the antioxidant capacity can be observed. Consequently, damage to erythrocytes by free radicals may occur. In such case, sufficient activity of superoxide dismutase and glutathione peroxidase protects the blood against oxidative damage.11 it is useful to control the alteration of total antioxidant status and lipid peroxidation in stored blood at the different days. Storage lesions cause changes in the red cell that are of two types; Metabolic - those disrupting the intracellular machinery of the red cells. Structural - those affecting the integrity of the cell membrane. Metabolic changes include following parameters like Adenosine triphosphate (ATP), 2, 3 DPG diphosphoglycerate (2,3 DPG), blood glucose, methemoglobin, reduced glutathione and certain enzymes. As discussed earlier, enzymatic cellular defence mechanism is provided by enzymes like glutathione peroxidase, catalase and other related enzymes of glutathione such as glutathione reductase and glutathione synthetase. Any decrease in the antioxidant defence capability of an oxygen consuming system or increase in reactive molecules in the system leads to oxidative stress. Thus, oxidative stress implies that there is a natural balance between free radicals and antioxidant defence and cells are damaged when the antioxidants are depleted or the radical formation is increased beyond the defence capacity of the cell. There are only few reports on the oxidative damage. The present work is an attempt to study the oxidative defects in terms of lipid peroxidation and some antioxidant enzymes which may develop during preservation.
MATERIALS AND METHODS The present study was carried out in the department of Biochemistry of KEM Hospital after obtaining the sanction of the hospital Ethics committee. For the present study, blood samples were collected from healthy voluntary blood donors. In this study, healthy voluntary blood donors and not the patients with any clinical disorders are not involved hence no additional clinical investigations were conducted in these volunteers. At the time of blood donation about 50ml of blood was separately collected in satellite bag out of the three bags of a collection set. The samples were preserved at 40C±20C in citrate phosphate dextrose adenine (CPDA) preservative in cold room of blood bank for thirty days. Aliquots were drawn from the bag on the 1st day, 8th day, 15th day and 30th day of storage. All the under mentioned parameters were studied in each of 30 bags. Each bag was studied five times within a period of 30 days at an interval of 7 days. 30 healthy donors who were willing to donate their blood for this study purpose were selected based on fulfilling the inclusion and exclusion criteria. After explaining the nature, objectives and benefits of the study to individual donor, informed consent was obtained prior to their entry into the study. Major Inclusion Criteria
Major Exclusion Criteria
About 50ml of blood was separately collected in satellite bag out of 3 bags of a collection set at the time of blood donation. These samples were stored in CPDA preservative at 40C±20C for 30 days in the cold room of blood bank. On the 1st day, 8th day, 15th day, 22nd day and 30th day, aliquots were drawn from the bags of the volunteers. Study Plan Volunteer’s first underwent tests confirming negative result for HBsAg, HIV, VDRL. After this confirmation, following laboratory investigations were carried out;
All the investigations were carried out with red cell membrane. The membranes were isolated by Dodge’s method that uses hypotonic electrolytic solution in lysis of the red cells.
The present work was undertaken with a view to study oxidative stress and enzymatic oxidants during blood preservation at 40C. All the statistical calculations namely mean, standard deviation, students paired and unpaired test ‘t’ test and correlation of coefficient were done using SPSS 10.01. Unit definitions
RESULTS Summary of the biochemical parameters studied is presented below. Table 1: Extent of Lipid peroxidation occurred during storage
Day1vs Day 8- p > 0.05 NS; Day1vs Day 15- p > 0.05 NS; Day1vs Day 22- p < 0.05*, Day1vs Day 30- p < 0.001*** Extent of lipid peroxidation measured on all 30 days by the amount of Malondialdehyde (MDA) is given table and depicted in a graph below.
Graph 1: Extent of Lipid peroxidation occurred during storage
Table 2: Values of Lipid peroxidation and antioxidants during storage over a period of 30 days
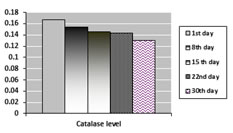
GP : Day1vs Day 8- p < 0.001***, Day1vs Day 15- p < 0.001***. Day1vs Day 22- p < 0.001***, Day1vs Day 30- p < 0.001*** Catalase: Day1vs Day 8- p < 0.01**, Day1vs Day 15- p < 0.001***. Day1vs Day 22- p < 0.001***, Day1vs Day 30- p < 0.05* From the data obtained in table 2, it is seen that the glutathione peroxidase level decreased from day 1 of storage upto day 30th. The decrease is statistically significant. Also indicates that catalase levels decreased from day 1 to day 30th. The decrease in levels of catalase on day 8th, day 15th, day 22nd and day 30th is statistically significant. Values of glutathione peroxidase and catalase levels in volunteers over a period of 30 days are shown in graphs below;
Graph 2 Graph 3 Graph 2: Values of Glutathione peroxidase (IU/ protein content) over a period of 30 days; Graph 3: Values of Catalase (IU/ protein content) over a period of 30 days Table 3: MDA levels over a period of 30 days Mean±SD in different blood group patients
Values expressed are mean±SD. NS : Not significant (P>0.05) From the data presented in the table 3, it can be observed that MDA level in stored blood decreased upto 15 days in blood groups of A+ve, B+ve, O+ve and AB+ve, but then there is sudden increase in its level. The decrease as well as increase is statistically non-significant. For B-ve, MDA level was constant upto 15 days after which it increased. Table 4: Value of Glutathione peroxidase over a period of 30 days in different blood group patients
Values expressed are mean±SD. NS : Not significant (P>0.05) It is evident from the above table that there is statistically non-significant decrease in glutathione peroxidase over a period of 30 days in blood groups of A+ve, B+ve, O+ve, AB+ve and B-ve. Table 5: Value of Catalase over a period of 30 days in different blood group patients
Values expressed are mean±SD. NS: Not significant (P>0.05) Above table 5 indicates that catalase levels decreased in blood groups of A+ve, B+ve, O+ve, AB+ve and B-ve over a period of 30 days which is statistically non-significant decrease.
DISCUSSION RBCs are more exposed to oxidative stress. The risk of oxidative damage among these cells is high but their antioxidant system is also more sensitive and powerful than other cells 17. In normal physiology conditions, there is a balance between RBCs’ antioxidant enzymes and free radicals18,19 but when erythrocytes are against oxidative stress, such as being in blood storage for a long period, RBCs’ antioxidant enzymes cannot protect erythrocytes against oxidative damage by free radicals. RBC storage lesion can occur due to oxidative damage, with a negative effect on RBC quality during storage. Lipid peroxidation, the oxidative deterioration of polyunsaturated fatty acids, is a common mechanism of cell injury and death. Although, earlier studies failed to detect MDA, a marker of lipid peroxidation in stored red cells, more recent investigations using sensitive techniques have found increased levels of this marker during red cell storage.20,21 Knight et al.22 observed a progressive and significant increase in mean MDA levels over 28 days of storage. The mean MDA on day 0 was 2.25 mmol/mg of protein and increased to 3.76 mmol/mg of protein on day 28 (p<0.005). Our study results are in agreement with earlier published data.23-25 Our study results indicate that there is an overall rise in the lipid peroxidation level throughout the storage period which suggest that the red cells yield to oxidative damage during preservation. Gaetani et al.26 published study results claiming catalase and glutathione peroxidase are equally active in detoxification of hydrogen peroxide in human erythrocytes. In this study, data indicates that throughout storage period, the levels of glutathione peroxidase and catalase declined. Statistically significant negative correlation existed between lipid peroxidation and glutathione peroxidase. This reduction in enzyme itself explains that the antioxidants produced are unable to protect the red cells from oxidative damage. Blood grouping of all the samples was done to check if there is any change in the levels of lipid peroxidation and antioxidant levels amongst the groups. The study shows that there was no significant susceptibility to lipid peroxidation when the different blood groups were compared, although some changes were marginally evident in O+ ve group.
CONCLUSION In the present study, slight increase in MDA level over a period of storage indicates lipid peroxidation of the red cell membrane during preservation. Antioxidant enzymes like glutathione peroxidase and catalase are active throughout the preservation period which act as safeguards. There was decrease in glutathione peroxidase and catalase levels during storage period indicating that negative correlation existed between lipid peroxidation and the enzymes. Red cell storage lesions due to oxidative injury during storage are now a well established fact, confirmed by the findings of the present study. Further studies are required to investigate the therapeutic role of antioxidants, either given to the donor before donation or added to the blood bag after red cell separation, in preventing oxidative damage to red cells during storage.
REFERENCES Willis, Robert. "Modern History Sourcebook: William Harvey (1578-1657): On The Motion Of The Heart And Blood In Animals, 1628". Fordham University.Hollingsworth MW: Blood transfusion by Richard Lower in 1665. Ann Med Hist 1928; 10:213–225.Landsteiner, Karl Zur Kenntnis der antifermentativen, lytischen und agglutinierenden Wirkungen des Blutserums und der Lymphe". Centralblatt f. Bakteriologie, Parasitenkunde u. Infektionskrankheiten, 1900;27: 357–362.Kohen, R., S. Chevion, R. Schartz and E.M. Berry, Evaluation of the total low molecular weight antioxidant activity of plasma in health and diseases: New approach. Cell Pharmacol. 1996; 3: 355-359.Boaz, M., Z. Matas, A. Biro, Z. Katzir, M. Green, M. Fainaru and S. Smetana, Comparison of hemostatic factors and serum malondialdehyde as predictive factors for cardiovascular disease in hemodialysis patients. Am. J. Kidnet Dis. 1999; 34: 438-444.Fiorillo, C., C. Oliviero, G. Rizzuti, C. Nediani, A. Pacini and P. Nassi, Oxidative stress and antioxidant defences in renal patients receiving regular hemodialysis. Clin. Chem. Lab. Med. 1998.; 36: 149-153.Gibson, J.G., R.D. Evans, J.C. Aus, T. Sack and W.C. Peacock, The post-transfusion survival of preserved human erythrocytes stored blood or in resuspension, after removal of plasma, by means of two isotopes of radioactive iron. J. Clin. Invest. 1974; 26: 715-738.Ross, J.F., C.A. Finch, W.E. Peacock and M.C. Sammons, 1947. The in vitro preservation and post-transfusion survival of stored blood. J. Clin. Invest., 26: 687-703.Orlina, A.R., A.M. Josephson, B. McDonald and J. Sobucki, 1969. Comparative viability of blood stored in ACD and CPD. Transfusion, 9: 62-69.Lewin, G. and I. Popov, The antioxidant system of the organism. Theoretical basis and practical consequences. Med. Hypotheses, 1994; 42: 269-275.Yamagchi, T., K. Uchimura, Y. Kurokova, M.M. Hamada, K. Inoue and N.Shibuya Selenium concenteration and glutathione peroxidase activity in plasma and erythrocytes from human blood. J. Clin. Biochem. Nature, 1992.12: 41-50.
Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 1978; 86: 271-278D. G. Hafeman, R. A. Sunde, W. G. Hoekstra Effect of Dietary Selenium on Erythrocyte and Liver Glutathione Peroxidase in the Rat The Journal of Nutrition 1974; 104(5),580–587Aebi H Oxygen Radicals in Biological Systems, catalase in vitro method. Enzymol 1984; 105:121-126.Claiborne A: Catalase activity . In: Handbook of method for Oxygene Radical Research. Greenwald RA(ed). CRC Press. Boca Raton. 1985; 283-4.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1-9.Sharifi S, Dzik WH, Sadrzadeh SM. Human plasma and tirilazad mesylate protect stored human erythrocytes against the oxidative damage of gamma-irradiation. Transfus Med. 2000;10(2):125-30.Wolfe LC. The membrane and the lesions of storage in preserved red cells. Transfusion. 1985;25:185–203.Bhargava AB, Pavri RS, Bhatia HM. Susceptibility of red cell to the oxidative injury during preservation. Indian J Med Res. 1988;87:202–5.Knight JA, Voorhees RP, Martin L, Anstall H. Lipid peroxidation in stored red cells. Transfusion. 1992;32:354–7.Anand AJ, Dzik WH, Imam A, Sadrzadeh SMH. Radiation induced red cell damage: role of reactive oxygen species. Transfusion. 1997;37:160–5.Sharifi S, Dzik WH, Sadrzadeh SMH. Human plasma and tirilazad mesylate protects stored human erythrocytes against the oxidative damage of gamma irradiation. Transfus Med. 2000;10:125–9.Cicha I, Suzuki Y, Tateishi N, et al. Gamma ray irradiated red blood cells stored in mannitol adenine phosphate medium: rheological evaluation and susceptibility to oxidative stress. Vox Sang. 2000;79:75–82.G. F. Gaetani, S. Galiano, L. Canepa, A. M. Ferraris, and H. N. Kirkman, “Catalase and glutathione peroxidase are equally active in detoxification of hydrogen peroxide in human erythrocytes,” Blood, 1989; 73(1) : 334–339.
Policy for Articles with Open Access
|
|
 Home
Home