Official Journals By StatPerson Publication
|
Table of Content - Volume 6 Issue 2 - May 2018
Levels of sialic acid, lactate dehydrogenase and g-glutamyl transferase in patients with carcinoma breast
M Gouri Devi1, Mohammed Abdullah Saad2*, A V Rukmini3, A Dhana Lakshmi4, Rama Rao5
1Associate Professor, 2Resident Specialist, 3Research fellow, 4Senior Specialist, Department of Biochemistry, Niloufer Hospital for Women and Children, Hyderabad, Telangana, INDIA. 5Professor and Head, Department of Biochemistry, Malla Reddy Institute of Medical Sciences, Hyderabad, Telangana, INDIA. Email: mohammadabdullahsaad@gmail.com
Abstract Background: Breast cancer is a malignant proliferation of epithelial cells lining the ducts or lobules of the breast. It is difficult to diagnose a patient in the early stages of the disease, therefore essential to develop investigations, which are cost effective and easy to perform at the primary care setup. The study is designed to assess the role of Sialic acid, Lactate dehydrogenase and γ-Glutamyl transferase in patients with carcinoma breast. Material and methods: This prospective randomised study was carried out on 60 female patients with carcinoma breast, divided into 3 groups. Levels of Sialic acid, Lactate dehydrogenase, γ-Glutamyl transferase were estimated in them, before and after treatment values are then compared with 90 controls. Results: The mean±standard deviation (SD) values of all the studied parameters were 420.22±73.44 for sialic acid; 92.98±55.94 for LDH and 37.34±40.71 for GGT. The values compared with controls and p value found to be significant (<0.001). The sensitivity, specificity and diagnostic accuracy was also calculated. Discussion: We have found significantly elevated total protein bound sialic acid levels in patients of breast cancer,there is an up regulation of LDH in the malignant cells also in patients with metastasis along with the elevation of GGT levels. Conclusion: We concluded, significantly elevated sialic acid levels are found in patients compared to controls, with 100% sensitivity and specificity. LDH and GGT levels are significantly elevated in patients and can be used for early diagnosis, staging and also to monitor the response of disease to the treatment. Key Words: Breast carcinoma; sialic acid; lactate dehydrogenase; γ-Glutamyl transferase
INTRODUCTION Breast cancer is a clonal disease. It represents a malignant proliferation of epithelial cells lining the ducts or lobules of the breast.1 A single transformed cell, as a result of acquired or genetic mutations, which are responsible for initiation and promotion of the disease, is able to express full malignant potential, this happens as a result of a series of events that occur in sequential and stochastic manner.1 Hence timely diagnosis and urgent management are essential. It is difficult to diagnose a patient in the early stages of the disease, therefore essential to develop investigations, which are cost effective and easy to perform at the primary care setup and to judiciously combine them with existing and regular markers to investigate the disease. Breast cancer is notorious for early spread to distant organs.1 Even after treatment there are cases, though less in number where patient approaches the doctor with a recurrence or an earlier unrecognized metastatic deposit. These patients can benefit a lot from new markers, which may be useful in predicting a recurrence and in recognizing metastatic deposit early. Some tumour markers, like the Estrogen receptor/ Progesterone receptor (ER/PR) status of the tumour are useful for staging the cancer and formulate appropriate treatment regime.2 But they are not affordable by the general population. New markers should be useful in assessment of early response to treatment. They also should be able to predict which patient with a benign disease may proceed to malignancy. The present study was designed to assess the role of certain markers in the diagnosis and staging of the disease and could also be used to predict the outcome of a particular treatment modality in patients. These are easily available and affordable markers, which requires minimum equipment and can be performed by minimally skilled personnel in the primary health care setup. Most tumour markers estimated for the detection of cancers are glycoproteins in nature. Markers used in breast cancer recognize a glycoprotein mucin expressed in the mammary epithelium, called episialin, which is identified by monoclonal antibodies.3 Episialin contains numerous sialic acid (N-Acetyl Neuramic Acid) residues, and the total sialic acid levels are elevated in breast cancer.The steady and consistent enzymatic milieu of a tissue is destroyed when it becomes malignant.4 Cellular enzymes are then released into the circulation and they can be detected in plasma. Lactate Dehydrogenase (LDH) and g-glutamyl transferase (GGT) are such enzymes, which are released into the circulation and can be easily measured.LDH is a hydrogen transfer enzyme that catalyses the oxidation of L-Lactate to pyruvate with the mediation of NAD+ as the hydrogen-acceptor. There are five isoenzymes of LDH present.5 LDH activity is present only in cytoplasm of all cells of the body. Concentrations in the cell are about 500 times greater than the levels in the plasma. Hence leakage from even a small mass of damaged tissue increases the observed activity to a significant extent. Patients with malignant neoplasms show elevated LDH activity in serum. Up to 70% of patients with liver metastasis and 20-60% of the patients with other metastasis show elevated LDH activity. All stages of breast cancer show increased activity of LDH.GGT catalyses the transfer of g-glutamyl group from compounds that contain terminal glutamyl residue attached. It is present in cytoplasm and membrane of the cells of proximal renal tubules, liver, pancreas and intestines. GGT levels are elevated in breast cancer patients with liver metastasis. GGT primarily comes from the liver, it is a sensitive marker of liver dysfunction. Changes in the cancer may be seen earlier and are more pronounced and can be used for superstaging of the disease.6 MATERIALS AND METHODS The study was conducted in the Department of Biochemistry, Osmania General Hospital and Medical College, Hyderabad. 60 female patients approaching and admitted to the Departments of Surgical, Medical and Radiation Oncology, Mehdi Nawaz Jung Institute of Oncology and Regional Cancer Centre (MNJIO and RCC), Hyderabad with carcinoma breast were included in the study. The patients were divided into 3 groups, based on the stage of the disease, stages II, III, IV. The levels of Sialic acid, Lactate dehydrogenase, γ-Glutamyl transferase were estimated in these patients, before and after treatment, and the values compared with 90 controls - premenopausal, postmenopausal and those with benign breast disease, 30 each. The patients were followed up for 7 days after surgery or 21 days after chemotherapy. Informed consent was obtained from all study participants from both cases and controls. General, systemic and local breast examination was done for all participants. 5 ml of venous blood was collected into plain tubes and allowed to clot. Serum was separated within one hour after collection. Care was taken to avoid haemolysis. Investigations
RESULTS In this prospective study, levels of various parameters in breast cancer was conducted over a period of 1 year in Department of biochemistry, Osmania general hospital. 60 female patients in various stages of breast cancer participated after giving their informed consent. The patients were from all social, economical and religious backgrounds. The study parameters were estimated in these patients and their levels were compared with those obtained in 90 female volunteers, also from all backgrounds, 30 of whom had a benign breast disease, and were included in the study as controls. The patients of the case groups were followed up and the same parameters estimated after treatment. 32 of patients underwent modified radical mastectomy and were followed up 7 days later, after wound healing and before they were discharged from the hospital. The remaining 28 patients had been administered and were followed up during their subsequent visit, 21 days later. Serum samples, free from hemolysis were used for the estimations. The values of Serum Sialic acid (SA) were expressed in mg/dl. The enzymes Lactate Dehydrogenase (LD) and γ-GlutamylTranferase (GGT) were expressed in the U/L. Results were statistically analyzed with the help of two software programs –
Two groups of controls, free from any kind of breast disease, were included in the study. All three parameters estimated were first compared among the three control groups and then compared with the cases. The means, standard deviations (SD) and results of t-test are in tables 1 and 2.
Table 1:
Table 2:
The cases, belonging to all stages and undergoing both kinds of treatment (surgery or chemotherapy) were put together and the mean and standard deviation (SD) values of all the studied parameters, before administering treatment, are presented in table 3.
Table 3:
The values of SA, LD and GGT were compared with those of all groups of controls put together, and data presented in table 4.
Table 4:
Mean of SA, LD and GGT were higher in the cases. The differences were significant in all cases when compared to all control groups. The sensitivity, specificity and diagnostic accuracy and the best cut off value of each investigation for breast cancer was also calculated. Comparison with all control groups are presented in the table 6
Table 6:
From the table above, SA is seen to be the best marker for detecting breast cancer, with a 100 % sensitivity as well as specificity. The diagnostic accuracy of SA is also high. The other markers have lower sensitivities and specificities compared to SA. In order to know the efficiency of markers in the staging of breast cancer, the means and SDs of these parameters were compared among one another. The means and SDs are presented in tables 7,8 and 9. Table 7: Stage II
Table 8: Stage III
Table 9: Stage IV
Sensitivity, specificity and diagnostic accuracy for staging were also calculated. The t-values, probabilities, sensitivities and specificities are presented in tables 10 (stage II vs III), 11(II vs IV) and 12 (III vs IV).
Table 10:
Table 11:
Table 12:
To differentiate between stage II and stage III, SA is found to be a better marker followed by LDH. Serum LDH and GGT are found to be better markers to distinguish stage II from stage IV followed by SA. Serum GGT and LDH are found to be better markers, followed by SA, to discriminate stage III from stage IV. To see if the markers could detect early stages of cancer, we also analysed all the markers and their efficacy in detecting stage II of the disease. Mean and SDs are shown in table 13 and t and p values, when compared to those of controls are shown in tables 14.
Table 13:
Table 14:
Significant differences are seen for SA and LDH between mean of cases and controls. The sensitivity, specificity and diagnostic accuracy of all parameters in stage II patients are also evaluated and presented in the table 15.
Table 15:
All the patients were followed up after treatment to note if the said treatment is able to bring the levels of parameters to control levels. The paired t-test and probabilities are noted in table 16 and t and p values on comparing after treatment values with controls are shown in table 17.
Table 16:
Table 17:
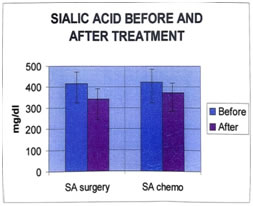
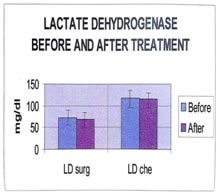
It is seen that the values of all parameters lowered on treatment, the decrease is significant in case of SA. Even after treatment, significant differences are seen between the analyte concentrations of cases and controls. The patients received two kinds of treatment- Surgery and Chemotherapy. The effects of these two modes of therapy on analytes were compared. The mean and SD of all parameters are shown in tables 18, 19. Tables 20, 21 show the results of t-test before and after surgery, and chemotherapy. Table 18: (Surgery)
Table 19: (Chemptherapy)
Table 20: (surgery paired t-test)
Table 21: (Chemotherapy paired t-test)
The differences in serum SA levels before and after treatment (surgery or chemotherapy) are significant. The levels of the analytes after treatment are compared with those of age-matched controls (post-menopausal), by using the unpaired student’s t-test, to evaluate if any of them have come back to control levels. The t and p values are presented in tables 22 and 23.
Table 22: (Surgery)
Table 23: (Chemotherapy)
Figure 1: Figure 2: DISCUSSION Breast cancer is the most common cancer effecting women in all urban areas of the world. Incidence and prevalence of the disease shows it affecting mostly older individuals.10,11 In our study, most patients were older, postmenopausal women, with few premenopausal patients. It is also known that nulliparity or having less number of children also predisposes a person to cancer. In accordance with epidemiological studies10,12 we have also seen that the mean number of children in cases was less than that of controls. Early studies in cancer cells showed that alterations take place in the cell surface molecules, and that these changes are essential for the expression of malignant phenotype of the transformed cell.13,14 Mostly glycoprotein or glycolipids act as cell surface molecules. Changes in cell surface molecules involve alterations in glycosylation of these glycoproteins.15,16 Sialic acid is the major end molecule associated with these cell surface constituents. Human breast produces and secretes sialic acid rich glycoproteins and gangliosides into the milk.6 This Production of sialic acid (normal during lactation) may serve as a specific tumour marker for breast cancer and needs to be further investigated. We have found significantly elevated total protein bound sialic acid levels in patients of breast cancer. This is in concordance with numerous other studies of increased sialic acid levels in breast cancer.17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33 We have demonstrated greater levels of sialic acid in advanced stages of the disease, may be because of spontaneous release of sialic acid rich glycoproteins and glycolipids takes place during the growth of a tumour cells, and its levels are related to the tumour burden and stage of the disease.17,20,22,23,24,25,26,29,30 Our study shows highest levels of sialic acid in patients with metastasis i.e. stage IV patients. Previous studies observed that sialic acid levels are detected in elevated concentrations about 8 years before the patients is detected clinically with the disease.34 Hence, the assessment of sialic acid levels may be indicative of the total pool of tumour markers circulating in the patients.We found a steep decrease in sialic acid levels in all groups of patients after treatment, whether chemotherapy or surgery (except the 2 non-responders), which can be useful to monitor the treatment.19 Metabolism of cancer cells is dependent more on glycolysis i.e. anaerobic respiration.35,36 There is an up regulation of LDH in these cells. LDH-M (isoenzyme) is known to be regulated by activation of estrogen receptor in breast cancer cells, by protein kinase C mediated pathways.37,38,39 This could be a more specific cause for elevated LDH in breast cancer. In addition to the 5 isoforms of LDH, another isozyme, LDC/LDX, which is present in the testis, is shown to be present cancer tissues, and the expression is similar to that of other germ-cell specific genes aberrantly expressed in cancer.40 This is a novel enzyme and is restricted to cancer cells. LDH is also a player in the oxidant-antioxidant status of tissues. In cancerous tissues, due to excess growth, large numbers of cells are destroyed and there is an increase in the levels of LDH.41,42,43,35,40 The greater dependence on glycolysis and excess cell destruction are the main causes for elevated LDH levels in serum of cancer patients. Cancers, which metastasize to the liver, cause a destruction of liver cells. LDH is a major liver enzyme and hence its levels increase, we have shown higher levels of LDH in patients with metastasis.42,5 We have also, shown LDH levels to be a discriminatory marker for staging of breast cancer, discriminating stage IV from stage II and III. Levels of LDH are significantly elevated in stage II patients, when compared to controls. Previous studies have shown similar results.44,45,46 On treatment of breast cancer, we have found no significant decrease of LDH levels. One of the other enzymes elevated in breast cancer is GGT. It is present in the cell membranes and acts in the metabolism of glutathione and other thioredoredoxins and glutaredoxins, which are important antioxidant defences of the body.5 In addition, it is present in greater levels in breast tissue, and optimal activity is essential for proper milk protein production.35 In the present study, GGT levels are increased cancer patients, compared to controls and is concurrence with other studies.47,48,6 GGT is over expressed in some clones of breast cancer.GGT provides cysteine to cancer cells in vivo, and this may be essential to metastasizing cells.49 There is a significant increase in stage IV disease when compared to stage II and III. The sensitivity and specificity of GGT are the highest among all the study parameters, to recognize stage IV. GGT can be used for staging cancer of the breast and previous studies have shown that elevated GGT is the best marker for detecting liver metastasis in breast cancer,50,42,6 the elevations starting about 8 months before clinical detection.
CONCLUSION From this study, we concluded that significantly greater elevations of sialic acid are found in cancer patients compared to controls. Excess sialysation of glycolipids and glycoproteins may account for its spontaneous release into the extracellular matrix. It is the best marker for the diagnosis of the disease with 100% sensitivity and specificity. It is a relatively good marker for staging. Significant decrease is observed after treatment, indicating patient response to treatment. Lactate dehydrogenase levels are also significantly elevated in breast cancer due to increased glycolysis of tumour cells and in liver metastasis.g-glutamyl transferase levels are also significantly elevated in cancer patients, may be due to destruction of breast cells, which contain the enzyme for normal physiological functions, or from micro metastasis to the liver. These 2 enzymes are found to be the best markers to differentiate stage IV from other stages. There is no significant difference in the enzyme levels after the treatment, probably due to shorter duration of follow-up.
REFERENCES
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home