Official Journals By StatPerson Publication
|
Table of Content - Volume 6 Issue 3 - June 2018
Duration of type 2 diabetes mellitus as a threat indicator for the microalbuminuria; A signal for diabetic nephropathy
Basavaraj Savadi1, Archana H B2*
1Assistant Professor, Department of Biochemistry, Basaveshwar Medical College and Hospital, Chitradurga, Karnataka, INDIA. 2Assistant Professor, Department of Biochemistry, D. Y. Patil Medical College and Hospital, Kolhapur, Maharashtra, INDIA. Email: bvsavadi69@gmail.com, archana_hbpatil@yahoo.co.in
Abstract Background: Diabetes mellitus is one of the major health problem leading to the long term kidney damage and responsible for 30-40% of all chronic kidney diseases. Aim and Objectives: The study was conducted to find the association of microalbuminuria in relevance to the duration of diabetes mellitus and HbA1c in a randomly selected type 2 diabetic patients. Material and Methods: The study was done from December 2012 to November 2013 in Raichur Institute of Medical Sciences, Karnataka. We have taken hundred type 2 diabetic cases based on history, clinical examination and related investigations. Result: Microalbuminuria contains a extremely significant correlation with the duration of diabetes (r = 0.81, p< 0.001) and HbA1c level (p = 0.001, r = 0.574). Conclusion: Relationship between microalbuminuria and the duration of diabetes mellitus shows more significant correlation compare to microalbuminuria and HbA1c level. The study has put light on the long term vulnerability of the high blood glucose as a risk for the accumulation of advanced glycosylation of end products which contribute the pathology to develop microalbuminuria rather than the HbA1c level alone. The present study emphasizes the need of regular screening for microalbuminuria and HbA1c, and can act as a risk indicator of diabetic nephropathy and early intervention to prevent complications. Key Words:, Diabetic nephropathy, Duration of diabetes mellitus, Glycated haemoglobin, Microalbuminuria.
INTRODUCTION Diabetes mellitus is one of the most challenging health problem in 21st century. Type 2 diabetes consists of 85-95% of all diabetes mellitus cases in developed countries. Worldwide millions of people were suffering from diabetes. More cases of diabetes is seen in adolescent to elderly age group. If timely intervention is not carried, the control on the diabetes will be out of hand1. Type 2 diabetes mellitus is a polygenic metabolic disease due to absolute or relative insulin deficiency condition resulting in hyperglycemia2. Type 2 diabetic patients usually have no symptoms of hyperglycemia for a long duration. As a result these patients are more prone to long term complications and are diagnosed at a late stage of the disease. Diabetes is a chronic diseases and sustained hyperglycemia attacks both micro and macro vessels throughout the body. Diabetic patients are more vulnerable to the long term complications related to eyes, kidney and cardiovascular system3. Glucose itself may have direct toxic effects on cells. Lorenzi et al4 have demonstrated cultured human endothelial cells that are chronically exposed to high glucose concentrations show important abnormalities in cell function, which cannot be ascribed to polyol pathway activity. Abnormalities include alteration in cell replication and maturation associated with evidence of damage to DNA. High glucose levels also lead to increased expression and synthesis of collagen, fibronectin and laminin, which may partly explain the enhanced products of extracellular matrix observed in diabetic kidneys. Mesangial cells in high glucose levels induce transcription and secretion of TGF-B, which is unique among the cytokines in that it stimulates the matrix synthesis and inhibits its degradation. Abnormalities in endothelial cell function have been implicated in the increased frequency of diabetic nephropathy. Poor glycemic control has been identified as one of the risk factors of microalbuminuria which hastens the progress of renal disease.5 Microalbuminuria is the excretion of albumin in urine ranging between 20 to 300mg/day6. Microalbumin in urine represents clinical marker for the dysfunction of endothelium of the kidney, which is one of the mechanism seen in diabetic nephropathy7. The commonest cause for the chronic renal damage is the nephropathy due to diabetes and accounts for the thirty percent of the Indian population. Its etiopathology being the interaction of polygenic causes like genetic and environmental factors.8 The early therapeutic intervention can be done to halt the progression of diabetic nephropathy towards the renal failure, as microalbumin in the urine can be detected much earlier than the full blown disease. The present study was conducted to work out the association of microalbuminuria in relevance to the duration of diabetes mellitus and HbA1c in a randomly selected type 2 diabetic patients.
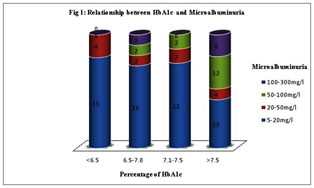
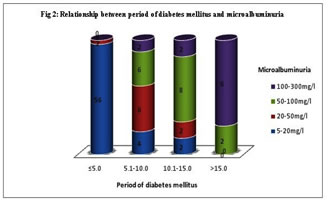
MATERIAL AND METHODS The study was done from December 2012 to November 2013 in Raichur Institute of Medical Sciences, Karnataka, attending medicine OPD. We have taken hundred type 2 diabetic cases based on history, clinical examination and related investigations. The random method was implied to choose the diabetic cases. Institutional ethical clearance and consent from the study group was taken. Sample Collection and Analysis: Fasting blood sample was collected for the estimation of HbA1c. HbA1c was estimated by Latex agglutination inhibition method using RANDOX KIT – HA 3830 in the Randox Daytona auto analyser. Microalbumin in urine is assessed by taking first morning sample in a clean container, discarding first and last few drops of urine. Microalbumin in urine was estimated by immunoturbidimetric method using RANDOX KIT – MA 2426 in Randox autoanalyzer. Statistical Analysis: Data was analyzed using SPSS software 19 version. Student t- test and pearson´s correlation was used to analyse the significance and correlation between the study parameters. (Figure no 1 and Table no 1) reveals that, among hundred diabetic patients, four diabetic patients out of twenty with HbA1c lower than 6.5 %, six patients out of twenty two with HbA1c between 6.5% to 7%, four patients out of sixteen with HbA1c between 7% to 7.5% and twenty four patients out of forty two with HbA1c more than 7.5% confirmed microalbumin in urine respectively. This shows that HbA1c has direct relation to the incidence of microalbuminuria and the finding is statistically significant (p=0.001) with mean ±SD is 8.12±1.44. (Figure no. 2 and Table 1) reveals that, fifty eight patients were categorized under the five years duration of diabetes, of that two were confirmed for mild grade microalbumin in urine. Twenty patients were having diabetes since five to ten years, of that eight, six and four patients were confirming mild, moderate and sever microalbuminuria respectively. Fourteen patients were having diabetes since ten to fifteen years, of that two, eight and four patients were confirming mild, moderate and sever microalbuminuria respectively. Among 8 patients with duration more than 15 years, two and six diabetic patients were positive for moderate and severe microalbuminuria respectively. This explains the statistically significant positive correlation ( p<0.001, r= 0.81) with mean±SD 6.36±4.60 between the relationship of duration of diabetic mellitus since diagnosis and microalbuminuria.
DISCUSSION Diabetes is the universal risk factor for the chronic renal damage9-12. Type 2 diabetes is characterized by the endothelial dysfunction, the extent of renal damage depends on the uncontrol level of glucose and the duration of exposure to the disease. Thus, the level of hyperglycemia and duration of exposure determines the non-enzymatic glycosylation of cellular proteins in diabetic nephropathy. The exposure of lysine amino terminal groups of protein to high glucose concentrations lead to increased covalent bonding of glucose to proteins. These covalent products can then participate in cross-linking between or within the protein molecules producing advanced glycosylation end products (AGE). This may impair the structural or enzymatic functions of the proteins and also affect their process of turnover and clearance. So causing AGE to accumulate in the tissues. The extent of glycation is dependent on proteins half-life, mean glucose levels and duration of exposure to the diabetes13. Percentage of HbA1c indicate the level of glycemic control. Poor glycemic index in type 2 diabetes mellitus serves as the causative factor for the ongoing pathogenesis in the disease. The HbA1c level reveals the mean glucose levels over previous 10-12 weeks. It is unaffected by recent food intake or recent changes in blood sugar levels. Reduction in 1% HbA1c will decrease long term complications to an extent of 30%.14 Microalbuminuria serves as the sensitive and early marker for the diabetic nephropathy15. Microalbumin in urine can be categorized into different levels mild (twenty-fifty milligrams/decilitre ), moderate (fifty-hundred milligrams/decilitre ) and severe (hundred –three hundred milligrams/decilitre)16-18. Microalbuminuria act as a distress signal for the imminent nephropathy. Our study revealed almost thirty eight percent of the study subjects shows microalbumin in urine. The past researches also shows the occurrence of microalbuminuria at the extent of 25% to 35%19-2). Our study shows HbA1c level with 8.12±1.44, and have significant relationship with microalbuminuria (p=0.001) (Table no 1). A threshold effect of hyperglycemia on the development of microalbuminuria was found. (Fig.1) shows, not much changes in the level of microalbuminuria with HbA1c lower than the 7%. The incidence of severe microalbuminuria rose steeply with increasing levels of HbA1c above 7.5%. Microalbuminuria is an earliest indicator of diabetic nephropathy. It is associated with poor glycemic control and the present study also concluding the same. Our study is in accordance with the study done by Naveen P et al.23 reveals the noteworthy relation between microalbumin in urine and glycated hemoglobin. Idogun ES et al.24 in their study showed the mean HbA1c was the highest in diabetic with microalbuminuria when compared with diabetic without microalbuminuria. The studies conducted by Huraib et al25 and Afkhami AM et al26 shows contradictory results, where there is no noteworthy relation between microalbumin in urine and glycated hemoglobin in diabetes patients. From the (figure no. 2), it is clear that there is high degree correlation of duration of diabetes with the microalbuminuria. It explains, longer the duration of the diabetes greater is the degree of microalbumin in urine which serves as a marker for the renal damage. Our research shows that diabetic patients who were exposed to the uncontrolled glucose level for more than fifteen years were more prone to develop full blown diabetic nephropathy, compare to the patients exposed to the diabetes for five to ten years. This suggests that there is strong and direct correlation between degree of microalbumin in urine and the duration of diabetes with mean±SD 6.36±4.60 ( p<0.001, r = 0.81 ) (Table no.1). Our study goes accordance with the previous studies, Huraib et al25, Varghese et al27, Mather et al28, which also suggests there is noteworthy relation between microalbumin in urine and the duration of diabetes Table 1: Assessment of different parameters and their significance with the microalbuminuria
*Significant association
Figure 1: Relationship between HbA1c and Microalbuminuria
Figure 2: Relationship between the duration of diabetes mellitus and the Microalbuminuria
CONCLUSION Relationship between microalbuminuria and the duration of diabetes shows more significant correlation compare to microalbuminuria and HbA1c level. The study has put light on the long term vulnerability of the high blood glucose as a risk for the accumulation of end products produced by the advanced glycosylation process which contribute to the pathogenesis behind the renal damage and development of microalbuminuria rather than the HbA1c level alone. The present study emphasizes the need of regular screening for microalbuminuria and HbA1c. Thus can act as a risk indicator of diabetic nephropathy and early intervention to prevent complications. REFERENCES
|
 Home
Home