Official Journals By StatPerson Publication
|
Table of Content Volume 12 Issue 2 - November 2019
Comparing tuberculosis treatment outcome of daily dose regimen versus intermittent- three times a week dose regimen of dots chemotherapy under RNTCP: A retrospective study
Shinde P M1, Holambe V M2*, Nagaonkar A S3
1Junior Resident, 2Associate Professor, 3Professor & HOD, Department of Community Medicine, GMC Latur, Maharashtra, INDIA. Email: pratapmshinde@gmail.com
Abstract Background: Tuberculosis (TB) is contagious and air-borne disease. Revised National Tuberculosis Control Program (RNTCP) adopted DOTS strategy since 1997. In 2017 the RNTCP had introduced daily regimen for treatment of TB across the country in place of the intermittent – three times a week dose regimen. Primary objective of this study was to compare the treatment outcome of the recently introduced daily dose regimen and the previous Intermittent- three times a week dose regimens of the directly DOTS. Study tools used were RNTCP tuberculosis (TB) registers at Tuberculosis Unit and quarterly reports on the ‘result of treatment of tuberculosis patients registered 13-15 month earlier’. It was a retrospective, record based analysis of treatment outcome of all tuberculosis patients registered at 9 tuberculosis units in district during 2nd quarter of 2016 for intermittent- three times a week dose regimen and all patient registered at same tuberculosis units during 2nd quarter of 2017 for daily dose regimen of directly observed treatment short course was studied. Results: Total 477 tuberculosis patients were registered at 9 tuberculosis units in district during 2nd quarter of 2016 for intermittent- three times a week dose regimen and there were 432 patients registered at same tuberculosis units during 2nd quarter of 2017 for daily dose regimen of directly observed treatment short course under RNTCP. Treatment success (favorable outcome) of daily dose regimen and intermittent- three times a week dose regimen of DOTS was 356 (82.6%) and 363 (77.2%) respectively. This difference was statistically significant at 95% confidence interval (p-0.04). Un-favorable treatment outcome of daily dose regimen– patient died-31(7.1%), treatment failure-1(0.2%), defaulted-40(9.3%), switched to catogery-IV-3(0.7%) and Un-favorable treatment outcome intermittent- three times a week dose regimen of DOTS- patient died-34(7.2%), treatment failure-16(3.4%), defaulted-55(11.7%), switched to catogery-IV-2(0.4%). One patient on daily and 7 patients on intermittent regimen were transferred out (outcome not evaluated) were excluded from comparison There was statistically significant (p-0.004) decline in failure rate in daily dose regimen as compared to intermittent- three times a week dose regimen of DOTS. Conclusions: ‘Treatment success’ rate is significantly higher with recently introduced daily fixed dose DOTS regimen than previous intermittent-three times a week regimen for treatment of tuberculosis under RNTCP. Key Word: Treatment outcome, comparison, daily dose regimen, intermittent regimen, DOTS.
INTRODUCTION Tuberculosis (TB) is contagious and air-borne disease. TB is one of the top 10 causes of death worldwide1,2. As per the Global TB report 2017 the estimated incidence of TB in India was approximately 2800000 accounting for about a quarter of the world’s TB cases3. Revised National TB Control Program (RNTCP) adopted Directly Observed Treatment Short-course (DOTS) strategy with intermittent three times a week regimen since 19974,7. Many researchers across the world concluded in their study that, daily dose regimen had certain advantages over intermittent two or three times a week regimen for treatment of tuberculosis8,14. In 2016, WHO had recommended that, the patients with drug susceptible pulmonary TB, the use of intermittent dosing is not recommended in both the intensive and continuation phases of therapy, and daily dosing is the recommended dosing frequency15. RNTCP adopted these recommendations immediately3, but there is still confusion whether the daily regimen or the intermittent regimen is superior. In our study, we have tried to fill up this lacuna.
OBJECTIVES Primary objectives of the study were to compare treatment success rate & to compare unfavorable treatment outcomes of the recently introduced daily regimen and the previous intermittent regimens of the DOTS under RNTCP.
METHODOLOGY Study tools: RNTCP TB registers at Tuberculosis Unit and quarterly reports on the result of treatment of tuberculosis patients registered 13-15 month earlier. Study design: A Retrospective, record based analysis of treatment outcome of Tuberculosis patients registered at 9 Tuberculosis Units. Study population and methods: Treatment outcome of all Tuberculosis patients registered at 9 tuberculosis units in district during 2nd quarter of 2016 (i.e. from 01.04.2016 to 30.06 2016) for Intermittent- three times a week dose regimen and all patient registered at same tuberculosis units during 2nd quarter of 2017 (i.e. from 01.04.2017 to 30.06 2017) for Daily dose regimen of Directly Observed Treatment Short Course was studied. Data was collected by visiting all TUs after obtaining clearance from institutional ethical committee, from RNTCP tuberculosis (TB) registers and quarterly reports on ‘Result of treatment of tuberculosis patients registered 13-15 month earlier’ available at Tuberculosis Units. Exclusion criteria: All patients registered for Category- IV and Category-V DOTS regimen for MDR and X-DR patient respectively and those patients registered for Non DOTS regimen for tuberculosis treatment were excluded from study. The following standard case and outcome definitions1,3,16,17 were adopted and used for the study. Successful treatment outcome: A treatment that ends up in cure or treatment completion
Treatment outcomes:
Data analysis- The analysis was carried out using the SPSS/PC Windows version 21.0 software package (IBM, Inc.). The results were compared by using the chi square test and Z- test.
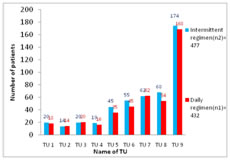
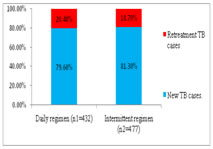
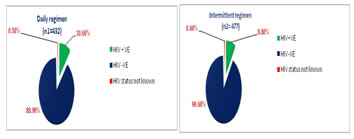
RESULTS AND DISCUSSIONS Total 477 tuberculosis patients were registered at 9 tuberculosis units in district during 2nd quarter of 2016 (i.e. from 01.04.2016 to 30.06 2016) for intermittent- three times a week dose regimen and there were 432 patients registered at same tuberculosis units during 2nd quarter of 2017 (i.e. from 01.04.2017 to 30.06 2017) for Daily dose regimen of directly observed treatment short course under RNTCP (Fig-1). Maximum number of cases was registered at TU No. 9, which covers population of Municipal Corporation area. Figure 1: TU wise No. of cases registered under DOTS In our study, we found that, the proportion of retreatment cases was increased to 20.4% in 2nd quarter of 2017, which was 18.7% in 2nd quarter of 2016(Fig-2). As per India TB report 20183, proportion of retreatment cases in India were 21.4%. Similar observation were noted in Report of first national anti tubercular drug resistance survey India 2014-16.18 Figure 2: Percentage of New and Retreatment TB cases In this study we observed that, the proportion of TB patients tested for HIV was 99.4% and 99.5% in 2016 and 2017 respectively. The proportion of TB patients positive for HIV was increased to 10.6% in 2017, from 8.8% in 2016 (Fig-3). As per India TB report 20183, percentages of TB patient tested for HIV were 88% in 2016. Percentage of TB patients positive for HIV was 4% at National level and 7% in Maharashtra.3,18 Figure 3: HIV status of patients registered under DOTS Treatment success (favorable outcome) of daily dose regimen and intermittent- three times a week dose regimen of DOTS was 356 (82.41%) and 363 (76.1%) respectively. (Table-1), this difference was statistically significant at 95% confidence interval (p-0.04). Table 1: Favorable and Unfavorable treatment outcome of TB cases
Un-favorable treatment outcome (Table-2) of daily dose regimen – patient died-31(7.1%), treatment failure-1(0.2%), defaulted-40(9.3%), switched to catogery-IV-3(0.7%) and that of intermittent- three times a week dose regimen of DOTS- patient died-34(7.2%), treatment failure-16(3.4%), defaulted-55(11.7%),switched to cat IV 2(0.4%). Outcome ‘Transferred out’ (outcome not evaluated) were excluded for comparison
Table 2: DOTS Treatment outcome of TB cases
No statistically significant difference in ‘treatment success’ of daily regimen and intermittent regimen of DOTS in HIV + Ve TB cases, this may be due to HIV status of patient (Table-3). Table 3: DOTS Treatment outcome of HIV positive TB cases
Table 4: DOTS Treatment outcome of HIV negative TB cases
In case of HIV –Ve cases the favorable treatment outcome of daily dose regimen was (84.7%), significantly higher as compared to intermittent three times a week regimen (79.0%). X2 4.4 and p value 0.03, statistically significant at 95% CI.
CONCLUSISONS We compared data of one quarter and came to a conclusion that, ‘Treatment success’ rate was significantly higher and ‘Treatment failure rate’ was significantly lower with recently introduced daily dose DOTS regimen than previous intermittent regimen. Treatment outcome cure with daily regimen was 31.8%, which was 36.8% with intermittent regimen; although this difference was not significant, data of one year of bacteriological positive cases need to be compared to get more details. No statistically significant difference in ‘treatment success’ of daily regimen and intermittent regimen of DOTS in HIV + Ve TB cases.
REFERENCES
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home