Official Journals By StatPerson Publication
|
Table of Content-Volume 7 Issue 3 - September 2018
Study on the role of streptococcus mutans in dental caries
Tasneem Fatima1*, Syed Salman Hamid Hashmi2, Abdul Qadir A W Siddiqui2, Humera Q F Ansari3
1Dental Surgeon, Mahavir Hospital and Research Center, Hyderabad, Telangana, INDIA. 2General Physician, Deccan College of Medical Sciences, Hyderabad, Telangana, INDIA. 3Apollo Institute of Medical Sciences and Research, Hyderabad, Telangana, INDIA. 4Professorand HOD, Department of Microbiology, Shadan Institute of Medical Sciences, Hyderabad, Telangana, INDIA. Email: ftasneem1994@gmail.com
Abstract Dental infections like tooth decay and periodontal disease are common chronic infectious diseases in humans. The mutans streptococci are found in plaque and are cariogenic. They colonize the host only after the eruption of the first teeth localizing the tooth surfaces and found in abundance in plaques of initial lesions. They are almost always recovered on cultivation of both initial and established carious lesions sites. A total of 48 children aged 6–18 years, were included in the study. Salivary samples were collected with a wooden spatula. A sample of chewed gum was also taken. From enamel, white spot and cavitated lesions, the plaque was obtained by swiping the tooth surface with a dental explorer. The samples were seeded on the surfaces of SB – 20M culture medium selective for MS sugar fermentation tests were done. The study group consisted of 24 children with dental caries and the control group had the same number, that is 24 children without caries. The study group had a culture positivity of 17 (70.8%), as compared to only 2 (8.3%) culture positives in the control group. Chewing gum was a better specimen as compared to saliva in our study which detected 17 (70.8%) of S. mutans versus 13 (54.2%) with sputum. Public health promotion of strategies to reduce colonization of mothers with streptococcus mutans, by use of xylitol, restriction of sucrose, filling of carious lesions and antiseptic treatment will prevent transmission to their children. The similar steps can be taken to improve the dental health of children also. Further studies are imperative to give an insight into the contribution of various risk factors in tooth decay. Key Word: Dental caries, Dental plaque, Mutans Streptococci (MS), Streptococcus mutans, Tooth decay
INTRODUCTION Dental infections like tooth decay and pericoronitis disease are common chronic infectious diseases in humans1,2. Dental decay is clinically measured on the tooth surface as a cavitation. The lesion starts as a sub-surface demineralization followed by a clinically detectable sub-surface lesion, the white spot and ultimately ends as cavitation, a late event in the pathogenesis of tooth decay3. The major hypotheses for etiology of dental caries are the specific plaque hypothesis, the non-specific plaque hypothesis and the ecological plaque hypothesis4,5,6. The specific plaque hypothesis stated that the species streptococcus mutans and streptococcus sobrinusare specifically responsible for active disease. According to the non-specific plaque hypothesis caries occurs due to the activity of the total plaque microflora consisting of many bacterial species5. The ecological plaque hypothesis proposes that caries is due to a shift in balance of the resident microflora brought about by changes in local environmental conditions6. The mutans streptococci are those streptococci which are found in plaque and are cariogenic7. They ferment mannitol and sorbitol and produce extracellular glucans from sucrose8. Clarke isolated these organisms from arious lesions in humans and named them S. mutans because on gram stain they were oval and appeared to be a mutant form of a streptococcus9. Dental decay occurs within months to a few years after eruption of teeth on certain teeth at specific sites10,11. Therefore, bacteriological studies pertaining to the etiology of tooth decay are performed best in children and young adolescents. Plaque samples are removed usually from fissures, approximal contact points or white spot lesions visible on the smooth surfaces.
Figure 1: Saggital (a) and cross-sectional (b) sections through a permanent molar. D, Distal; M, mesial; B, buccal; L, lingual surface. Note fissure decay (shaded area midway down in fissure) and approximal decay (subsurface shaded area on distal surface)
Mutans streptococci colonize the host only after the eruption of the first teeth12,13, localizing the tooth surfaces and found in abundance in plaques of initial lesions14,15. Their virulence expression is linked with carbohydrate consumption, especially sucrose16,17. They synthesize from sucrose certain macro-molecules that aid in their attachment to the teeth18,19. They produce acid rapidly from simple carbohydrates like sucrose, and are tolerant to low PH 20,21. They are almost always recovered on cultivation of both initial and established carious lesion sites22,23.
MATERIALS AND METHODS A. Subject Population A total of 48 children aged 6 – 18 years, who had not taken antibiotics in the past 10 days were included in the study which was conducted in the Department of Microbiology at Shadan Institute of Medical Sciences. Study conducted from January 2018 to June 2018. Written consent was obtained from the parents/guardians. B. Sampling 2 hrs after tooth brushing non-stimulated salivary samples were collected with a wooden spatula during 1 min (Kohler and Bratthall, 1979)24. A sample of chewed gum was also taken. After 10 min of chewing, 50-70% of the detected species in the salivary and adheringmicrobiome are ultimately detected in the chewed gum piece25. From the caries subjects, plaque was collected separately from each of the following four different types of sites.
From Enamel, white spot and cavitated lesions, the plaque was obtained by swiping the tooth surface with a dental explorer. The carious dentin was excavated with either a spoon excavator or a round burr at slow speed using a dental drill. C. Culture The samples were seeded on the surface of plates containing SB – 20 M culture mediumselective for MS. The plates were incubated for 3 days at 37°C in a microaerophilic atmosphere using the candle jar technique. The SB-20 M medium of Davey and Rogers (1984)26 was modified with sucrose replaced by coarse granular cane sugar. Composition of the Medium per liter: Bacto-casitone –15g; yeast extract 5g; L-cysteine 0.2g; sodium sulfite 0.1g; sodium acetate 20g, coarse granular cane sugar 200g; 15g agar and distilled water. After autoclaving for 20 min at 120°C and cooling to 50°C, bacitracin was added to a final concentration of 0.2 U per ml agar. The medium was poured in plates, stored at 4°C and used within a period of 7 days. D. Identification of S. mutans Colonies of S. mutans showed a granular surface similar to ground glass, with or without a scintillant polysaccharide drop on the surface. Few colonies were transferred to tubes containing thioglycollate broth without glucose or indicator. The broth cultures were used as inoculum to test for the ability of each isolate to ferment mannitol, sorbitol, raffinose and melibiose27 and to produce Hydrogen peroxide28.
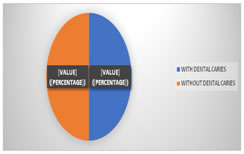
OBSERVATIONS AND RESULTS A total of 48 children all males between 6 – 18 years were included in the study. 24 (50%) of the children had dental caries (study group) and the rest that is 24 (50%) did not have any carious teeth (control group). (Fig 2)
Figure 2: Depicting the number of children with or without caries. According to the age group – the study group had 13 (54.2%) patients in the 6 – 9 years age group, 9 (37.5%) in the 10 – 13 years age group and 2 (8.3%) in the 14 - 17 years age group. In the control group there were 14 (58.3%) children among the 6 - 9 years, 9 (37.5%) among the 10 – 13 years old and 1 (4.2%) in the age group of 14 - 17 years (Table 1)
Table 1: Age-wise distribution of children in the study and control groups
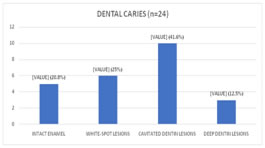
Among children with carious teeth, 5 (20.8%) had Intact enamel, 6 (25%) had white-spot lesions, 10 (41.7%) had cavitated dentin lesions and 3 (12.5%) had deep dentin lesions. (Fig 3)
In the study group out of the 13 in the 6 – 9 years age group, 10 (76.9%) yielded positive cultures when sampled from a chewing gum and 8 (61.5%) were positive with saliva. In the 10–13 year age group, out of the 9 students6 (66.6%) cultures were positive using a chewing gum specimen and 5 (55.6%) using saliva. In the 14–17 years age group, out of 2 students 1 (50%) wasculture positive with chewing gum and no culture positivity was seen with saliva. (Table 2)
Table 2: Culture positive for Smutans-using chewing gum and salivary specimens
In the control group only 1 (7.1%) specimen of chewing gum showed culture positivity from 14 students in the 6 – 9 years age group and 1 (11.1%) from the 9 students in the 10 – 13 years age group. No specimens of saliva from control group showed growth of streptococcus mutans. (Table 3)
Table 3: Culture positivity in the control group
DISCUSSION The data from our studies show that S. mutans are human adontopathogens. In the study group of 24 patients with dental caries, 17 (70.8%) showed a growth of S. mutans in contrast to the control growth also of 24 healthy individuals with no caries, showing a culture positivity of only 2(8.3%) specimens with growth of S. mutans. Other studies have also reported a 10 - 15% of caries active subjects that do not have detectable levels of S. mutans29. Aciduricity is a significant attribute of S. mutans that can be associated with its cariogenicity and selection in stagnant areas. Studies based on molecular techniques found S. mutans to be dominant in advanced caries30. Mutans streptococci are transmitted to humans in early childhood, after the first teeth erupt and they originate from their mothers, that is vertical, matrilineal transmission31,32. Our study group was consistent with these studies showing a relatively greater numberof children in the 6-9 year age group with caries. Colonization with mutans streptococci occurs soon after eruption of teeth and if the depths of the fissures become colonized, then decay becomes inevitable. However, if colonization is delayed until the depths are occupied by other bacteria, then possibly decay may not occur or be greatly reduced. In caries active patients, frequent sucrose consumption may be associated with the emergence of the mutans streptococci. In Indian culture, where sweets are an integral part of everyday meals and especially festive occasions, children are introduced to sucrose at a very early age and this continues into adolescence and adulthood. Our study group showed caries in a considerable proportion of children, with 10 (76.9%) positive for S. mutans in 6–9 years age group and 6 (66.6%) cultures positive in the 10–13 years age group. Chewing gum was a better specimen as compared to saliva in our study as detected 17 (70.8%) of S. mutans versus 13 (54.1%) with sputum. The use of xylitolchewing gum by Finnish mothers till their children turned 3 years inhibited the colonization of their children with S. mutans and reduced the caries incidence among them during a five year period of observation. There is a need for the development of better and more potent antimicrobial agents than chlorhexidine. Public health promotion of strategies to reduce colonization of mothers and caregivers by the use of xylitol, restriction of sucrose, filling of carious lesions and antiseptic treatment will go a long way in preventing caries among children.
CONCLUSION Evidence from current study supports the pivotal role of mutans group of streptococci in the initiation of caries in teeth of children and suggest strongly their etiological role in the induction of caries not only on smooth surfaces and fissures but also root surface caries. The understanding of the ecology of S. mutans helps in designing strategies to interfere with the colonization of S. mutans in turn decreasing the incidence of dental decay. Further studies are required to thoroughly understand the contribution of various risk factors like maternal colonization and use of confectionary with sucrose in between meals. Strategies to improve the dental health of both children and adults should be promoted.
REFERENCES
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home