|
Table of Content - Volume 15 Issue 3 - September 2020
Type II Diabetes and sensorineural hearing loss
Preeti S. Raga1*, Amrith Waghray Lal2, Shruti Mohanty3
{1Associate Professor, 2Professor, Department of ENT} {3Professor, Department of Biochemistry} Kamineni Institute of Medical Sciences, Kamineni Staff Quarters Kamineni Campus, Narketpally, Dist. Nalgonda, Telangana, INDIA.
Abstract Background: To compare hearing acuity in patients suffering from diabetes mellitus with normal subjects by pure tone audiometry. Objectives: 1. To assess hearing in Normoglycemic subjects and Hyperglycemic subjects by pure tone audiometry. 2. To compare the auditory acuity of diabetics and nondiabetic subjects. 3. To analyse whether the uncontrolled diabetes and the duration of diabetes mellitus, is related to the severity of hearing loss. Methods: A study was done with fifty type 2 diabetes mellitus patients between ages of 30-50 years attending the diabetic OP, and fifty non diabetic (Control) people matched with age and sex, attending the master health check-up scheme at Kamineni Institute of Medical Sciences Naketpally Nalgonda district Telangana, India. The following investigations such as fasting and postprandial blood sugar, glycosylated haemoglobin (HbA1C) and pure tone audiometry were carried out in all the patients. Results: The number of patients affected with sensorineural deafness among the diabetics were 88% when compared to that of the controls 8%, which is highly significant (P = 0.001). The audiograms of the diabetics were suggestive of mild to moderate sensorineural deafness and the hearing loss was more towards the higher frequencies. Conclusion: The diabetic patients showed significant high frequency, bilateral, mild to moderate sensorineural hearing loss (88%) as compared to the controls of similar age. The glycaemic status have significant correlation with the hearing loss but duration of diabetes have no significant correlation with hearing loss and both the sexes were equally affected. Key Word: Audiometry, HbA1c, Sensorineural hearing loss, Type 2 diabetic mellitus
INTRODUCTION Diabetes mellitus is the one of the endocrine disorder most commonly related with auditory diseases. The mortality and morbidity is mainly due to long term micro and macro vascular complications affecting the blood vessels of eyes, kidneys, heart and nerves1,24 and it has been postulated that the micro vascular complications also affect the hearing of diabetes patients. Studies have demonstrated thickening of the basement membrane of the capillaries of stria vascularise2 in diabetic animals. Histopathological studies have shown damage to the nerves and vessels of the inner ear of the individuals with diabetes. These vascular changes have been theorized to be an important causative factor for neuronal degeneration in the auditory system3,25. Few studies implicated diabetes mellitus as independent causative factor of sensorineural hearing loss4. Many different types of hearing loss has been found in diabetic patients; one of them is progressive, gradual bilateral sensorineural loss, affecting especially high frequencies and the elderly. It would be similar to presbycusis, but with more severe losses than those expected by aging5. Conversely, there are authors who report the possibility of having early sensorineural loss6. Some studies described diabetes as the possible cause of sudden hearing loss7. The probable mechanisms of hearing losses are microangiopathy of the inner ear, neuropathy of the cochlear nerve, a combination of both, outer hair dysfunction and disruption of endolymphatic potential. The following pathological changes were observed in diabetic patients with sensorineural hearing loss by various authors
MATERIALS AND METHODS 100 (Subjects) were classified into two Groups. Group I (n=50) subjects, Group II (n =50) type. Group I (n=50) Type 2 diabetic patients from Medical out patients and group II (n=50) non – diabetic healthy (control) people, matched with age and sex, visiting the master health check-up scheme at Kamineni Institute of Medical Sciences Naketpally Nalgonda district Telangana India, attended hospital between January 2018 to February 2019. Inclusion criteria were type 2 diabetic patients between age 30 to 50 years and non-diabetic, age and sex matched healthy subjects with no previous history of ear diseases. Exclusion criteria were family history of hearing loss, history of chronic suppurative otitis media, meningitis, head or ear trauma, history of ear surgeries performed in the past, and history of ototoxic drug intake, and systemic diseases like hypertension, cardiac diseases , renal failure and occupational noise exposure. Written consent was obtained from all the subjects enrolled in the study after explaining to them in detail about the study in their own language. The study was approved by the institutional Ethical committee Kamineni Institute of Medical Sciences Narketpally, Nalgonda district, Telangana. Hearing assessment was done by clinical history, ear examination including tuning fork tests and Pure Tone Audiometry (Audiometer model 500 MK III of Arphi company in a sound proof room in the ENT department of Kamineni Institute Of Medical Sciences Narketpally, Nalgonda district Telangana ) to determine hearing function, the degree, type and configuration of a hearing loss if any.
Biochemical parameters for diabetes The new cases were considered as diabetics when their fasting blood glucose value was > 126 mg/dL and post prandial plasma glucose was > 200 mg/dL. HbA1c level were determined in all the subjects. Data was statistically analysed using Pearson correlation coefficient. The subjects also underwent the following tests, Statistics: Student t test has been used to find the significance of auditory thresholds (dB) between various categories of parameters. Analysis of variance [ANOVA] has been used to find the significance of auditory thresholds in different groups. The statistical software namely SPSS 11.0 was used for the analysis of the data and Microsoft Word and Microsoft Excel have been used to generate graphs, tables, etc. Table.1. Compares the glycemic levels in groups I and II which is highly significant (P = 0.001). Using the Chi square test the number of people affected with sensorineural deafness among the diabetics is 88% (44/50) when compared to that of the controls (8%, 4/50) which is highly significant (P=0.001).
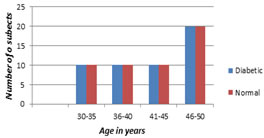
OBSERVATION AND RESULTS More number of patients was seen in 46-50 years of age group and 60% were females (Table 1 and 2)
TABLE I: Age distribution (N=100)
More number of diabetic patients were seen in 46-50 years of age group.
Graph I: Age distribution
TABLE=II: Sex distribution ( n=100)
40% of cases and controls are men and 60% are women.
Table III: Blood Sugar level (n=100)
Differences in the fasting blood sugar and post prandial blood sugar level between the cases and controls were clinically Significant. Patients with hyperglycemia have their levels of blood sugar about twice that of control.
Table IV: Sugar levels Compares the glycemic levels in groups I and II
The number of people affected with sensorineural deafness among the diabetics is 88% (44/50) when compared to that of the controls (8%, 4/50) which is highly significant (P = 0.001).
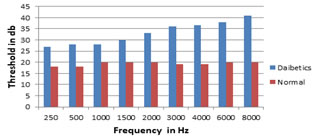
Table V: Effect of diabetes mellitus on auditory thresholds in db
*Statistically significant by student t test
Graph II: Effect of diabetes mellitus on auditory thresholds in db
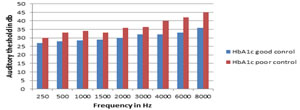
Table VI: Auditory thresholds (db) in HbA1c-wise subgroups of diabetics (Mean ± SD)
*Statisti
Graph III: Auditory thresholds (db) in HbA1c-wise subgroups of diabeticsAs shown in table and graph V, from 250-8000 Hz frequency, there was a significant difference between diabetic patients with good control of their blood sugars [HbA1c <6-8%] versus diabetic patients with poor control of their blood sugars [HbA1c >8%].The number of patients in good control group were 26 and the poor control were 24.
Table VII: Auditory thresholds (db) in duration-wise subgroups of diabetics
The effect of duration of diabetes on hearing threshold was not much significant clinically and statistically.
DISCUSSION This study has evaluated the hearing loss in the patients with diabetes and the influence of hyperglycemia on hearing and it shows that the diabetics had a 88% incidence of deafness when compared to the non- diabetics of the same age group (8%) (Table1). Other studies showed the incidence of hearing loss ranging from 30% to 95%. Friedman et al.13] showed a 55% incidence of hearing loss in diabetic patients. Kakarlapudi et al.14,24,25 found that hearing loss was more common in diabetic patients (13.1% prevalence) than the control. Weng et al.15 noted that among the 67 diabetic subjects examined, 44.8% of them had profound hearing loss. In his study,the duration of diabetes (above or below 10 years) was found to have no effect in the incidence of hearing loss in the diabetic group(Table VII), whereas the glycemic control was found to have effect in the incidence of hearing loss (HbA1C level above or below 8) (Table VI). Taylor and Irwin (1978)6 observed that female patients with diabetes had significantly greater hearing loss when compared with male patients with diabetes. According to Cullen and Cinnamond (1993)16, male patients with diabetes had worse hearing than female patients with diabetes. In our study we observed no differences between the gender (Table II). Many studies suggested that diabetes causes hearing loss.. In this study, the audiogram analysis of controls and diabetics showed that 8% of the controls had hearing loss (Table IV ) and 44 (88%) of the diabetics had hearing loss(Table V). In our analysis, hearing loss was observed at higher pure tone frequencies at 4000 to 8000 Hz. Audiograms of the diabetic patients had no significant air bone gap and the hearing loss was of the sensorineural type. This result was in concordance with previous studies16, 17,. But Fangchao Ma et al.18 and Friedman et al.12 observed the strongest association of hearing loss at lowest frequency at 500 Hz. Gibbin and Davis17 found a statistically significant incidence of type II tone decay in the overall group of diabetics at 2000Hz. According to Frisina et al.19 the greatest deficit of hearing in the diabetics tended to be at low frequencies. Vaughan et al.20 suggest that diabetic patients 60 years old or younger may show early high frequency loss similar to early presbycusis. Our study reports that the incidence of sensorineural deafness is increased in diabetics.(Table VI) The hearing loss is a progressive, bilateral, sensorineural deafness of gradual onset which affects predominantly the higher frequencies. The decrease in hearing acuity is similar to presbycusis but those affected show a hearing loss greater than could be expected at that age. Among diabetic patients, poorly controlled diabetic group [HbA1c >8%] having more hearing loss in all frequencies when compared with good control diabetic group [HbA1c< 6-8%] (Table VII)
CONCLUSION Though the sample size considered in this study was small, the study offers significant clinical implications. From the study, it was seen that majority of the Diabetes mellitus patients had subclinical hearing loss. The age of the patient and degree of glycemic control had a positive correlation with hearing loss. Gender and duration of diabetes did not have any effect on the hearing status. Health-care providers need to be aware of the coexistence of hearing symptoms and incorporate audiological referral as a routine practice in future for every patient with DM. Future studies are needed to understand the existence of central auditory processing deficits or auditory neuropathy in these individuals and to note the change in hearing threshold with an improvement in glycemic control.
Declaration: I declared that, in this study there is no conflict of interest, no Sponsorships, no funding done .Informed consent was obtained from all individual participants included in the study. Animal rights - not applicable. Plagiarism –not done/not crossed the limit. Ethical clearance from institutional Ethical comity.
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home
 cally significant
cally significant