|
Table of Content - Volume 13 Issue 2 - February 2020
Evaluation of perinatal outcome in early and late pregnancy haemorrhage in tertiary care centre
Alka Patil1, Shruti Singh2, Rahul Patil3*
1Professor and Head of Department, 3Senior Resident, Department of Gynaecology, ACPM, Dhule, Maharashtra, INDIA. 2Consultant Gynaecologist, Department of Gynaecology, Indira IVF Centre, Delhi, INDIA. Email: doctor.rvp@gmail.com
Abstract Aims and Objectives: 1To study perinatal outcome in early pregnancy hemorrhage.2)To study perinatal outcome in late pregnancy hemorrhage. Materials and Methods: 1. This is observational prospective study performed within span of 1 year (August 2014 to July 2015) in the Department of Obstetrics and Gynaecology in ACPM Medical college, Dhule. 2Total number of 200 women with bleeding per vaginal during pregnancy were enrolled in the study. The patients who presented with vaginal bleeding were divided into: 1Early pregnancy bleeding < 20 weeks, 2. Late pregnancy bleeding > 20 weeks Since the demographic profile, etiology, risk factors involved, maternal and perinatal morbidities and mortalities associated with early and late pregnancy group are drastically different, so the cases in this study are divided into two groups – EARLY (<20 weeks) and LATE (>20 weeks). Results: In this study, 66% patients presented with early pregnancy bleeding and 34% with late pregnancy bleeding. Perinatal outcome in Early pregnancy bleeding:- 30% spontaneous abortion, 7.6% preterm delivery, 4.5% IUGR. Perinatal outcome in Late pregnancy bleeding:- 3% stillbirth, 14.7% IUGR, 5.9% IUD, 58.8% preterm birth, 2.9% early and late neonatal deaths. Conclusion: Vaginal bleeding at any stage of pregnancy is an alarming event and can be potentially life- threatening to mother and fetus. Obstetrician should ensure that patient delivers in a well equipped centre with NICU facilities and expertization of neonatologist. Key Word: haemorrhage.
INTRODUCTION Pregnancy is an important but potentially stressful time in a woman’s life. When an unexpected, potentially dangerous event such as vaginal bleeding occurs, it is extremely upsetting for the expectant mother and father. While the obstetrician may not always be able to bring good news to these patients, in many cases early intervention can make the difference between life and death for the fetus, and at times the mother as well. Resuscitation of the mother, fetal monitoring, fetal gestational age estimates, an ultrasound to rule out previa as a cause, and obstetrical consultation should be performed in all cases. Early paediatric consultation is also essential when dealing with a viable fetus. Causes of early pregnancy bleeding: Haemorrhage in early pregnancy may be caused by abortion, ectopic pregnancy, trophoblastic disease, cervical carcinoma. It may be caused by pedunculated fibroid, cervical mucus polyp, cervical erosion or vulvar and vaginal lesions. Spotting in the first trimester may be a prelude to haemorrhage in the third trimester.1Vaginal bleeding after midpregnancy is associated with maternal and fetal risks. Maternal morbidity may be caused by acute hemorrhage and operative delivery, and the fetus may be compromised by uteroplacental insufficiency and premature birth.2 Causes of late pregnancy bleeding: Placenta pravia Abruptio placentae Distal genital tract/ gynaecological bleeding Abnormal placentation Abnormal placental shape Vasa previa3 In this study perinatal outcome in early pregnancy hemorrhage and late pregnancy hemorrhage are analysed.
AIMS AND OBJECTIVES
MATERIALS AND METHODS This is observational prospective study performed within span of 1 year (August 2014 to July 2015) in the Department of Obstetrics and Gynaecology in ACPM Medical college, Dhule. Total number of 200 women with bleeding per vaginal during pregnancy were enrolled in the study. The patients who presented with vaginal bleeding were divided into:
Inclusion criteria
Exclusion criteria
OBSERVATIONS AND RESULTS Table 1: Incidence of pregnancy affected by vaginal bleeding
Approximately 1 in every 6 pregnancies is affected with vaginal bleeding.
Table 2: Incidence of vaginal bleeding
Since the demographic profile, etiology, risk factors involved, maternal and perinatal morbidities and mortalities associated with early and late pregnancy group are drastically different, so the cases in this study are divided into two groups- EARLY (< 20 weeks) and LATE (>20 weeks), and studied separately in terms of perinatal outcome.
Table 3: Distribution of cases according to age of patient
Maximum number of cases were concentrated in 26 – 30 years of age with mean age of 28 years. Table 4: Distribution of cases according to gravidity
Maximum number of cases were multigravida.
Table 5: ANC care of early pregnancy bleeding
Table 6: ANC care of late pregnancy bleeding
Majority of cases were unbooked and booking status was minimal especially with early bleeding group. Table 7: Cases distribution of bleeding during pregnancy
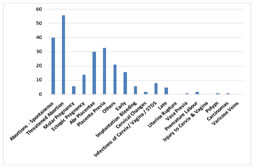
Various causes of vaginal bleeding during pregnancy are shown here. Maximum numbers of cases were of threatened abortion and minimum were of bleeding due to cervical changes in early pregnancy group. And among late pregnancy group, maximum cases were of placenta previa and minimum were of vasa previa, polyps and carcinoma.
Table 8: Gestational age at delivery in Early pregnancy bleeding
Table 9: Gestational age at delivery in Late pregnancy bleeding
In Late pregnancy bleeding group, preterm delivery rate was higher than term delivery.
Table 10: Perinatal outcome in Early pregnancy bleeding
Approximately 30% cases ended up in spontaneous miscarriage and the ones which continued till period of viability had many adverse perinatal outcomes in the form of IUGR, preterm delivery and NICU admissions. Table 11: Perinatal outcome in Late pregnancy bleeding
Above table shows high number of perinatal mortality in cases with bleeding in late pregnancy, both in-utero and ex-utero. NICU admissions were also found to be high among these cases.
Table 12: One minute APGAR scores among gravidas with Early pregnancy bleeding :-
Table 13: Five minute APGAR scores among gravidas with Early pregnancy bleeding
Only 72 cases out of total 132 cases, with early pregnancy bleeding, survived the period of viability.
Table 14: One minute APGAR scores among gravidas with Late pregnancy bleeding
Table 15: Five minute APGAR scores among gravidas with Late pregnancy bleeding
Due to perinatal asphyxia, most of the newborns had low APGAR scores at 1 and 5 minutes in late pregnancy bleeding cases as compared to early pregnancy bleeding group. Table 16: Complications among the live newborns in Early pregnancy bleeding
live newborns of early pregnancy bleeding group showed no significant increase in the complications as compared to late pregnancy bleeding group. Table 17: Complications among the live newborns in Late pregnancy bleeding
newborns of late pregnancy bleeding group suffered from a variety of complications of preterm delivery and perinatal asphyxia and so the rate of nicu admissions was also higher.
DISCUSSION Bleeding during pregnancy is a common clinical entity in our set- up. Whether occurring in early or later half of pregnancy, antenatal bleeding has significant impact on perinatal outcome. Distribution of various cases of bleeding during pregnancy reporting to our hospital shows the predominance of antepartum haemorrhage accounting for 31.5% of the cases, with an overall incidence of 5.6%; which corroborates by Fouzia et al 2010.4The high incidence of LPB, reported in this study, may be because of large number of referred cases to this tertiary care centre, but still this may be an underestimate of actual figure as many such patients fail to reach hospital in time or a multitude of cases do not report to any hospital at all. In EPB group, maximum number of cases were clustered in mothers of higher age group of 26 – 30 years ( 39%). Similarly in LPB group, maximum number of cases (48.3% ) presented in age group of 26- 30 years with a mean of 20.01 years while Fouzia et al 20104 reported a mean of 30 years, Savita et al 20085and Das et al 19756(26.8 years each). Other studies reporting such higher incidence of LPB in multipara (around 5-8 times than that in primi) are- Gilliam et al 20027,William et al 19938, Ananth et al 19969.Higher order parity was once again proved to be positive risk factor in LPB group (79.3% in multipara in present study, 92.3% in Fouzia et al 20104),Savita et al 20085 (63.01%).But this relation was not detected for EPB group. So, being a problem of multiparity, reduction in family size and the issues of contraception are highly applicable if this incidence has to be reduced. Majority of cases were unbooked, signifying the importance of ANC care and revealing the lacunae in the MCH care. Booking status was below the half level in EPB group (25%). But in cases of LPB, this relationship was milder (44%). This clearly shows the importance of antenatal care in prevention and early detection of antepartum haemorrhage to reduce morbidities and mortalities. There are studies that find normal postnatal development after threatened abortion (Buck et al 196910 and Barker et al 196711).However, some of the studies find that the association between bleeding in early pregnancy and suboptimal outcome may be useful for detecting pregnancies at risk. This was suggested by Hobel et al 197312 and Adelstein and Fedrickin 197813. The various cases of EPB group comprised predominantly of threatened miscarriage (28%), most of which crossed the period of viability with proper ANC care with favourable pregnancy outcome. The other important diagnosis were ectopic and molar pregnancies (7% and 3%) in EPB group. Placenta previa accounted for majority of cases in the late group (48.5%) against abruption (44.1%) and other causes (7.3%). 21.2% of threatened miscarriage continuing beyond 24 weeks ended in preterm delivery and 58.8% of LPB group cases had the same outcome. This finding alone may be responsible for the other morbidities such as low birth weight, depressed 1 and 5 minute APGAR scores and increased perinatal mortality. Various other studies which have postulated the increased risk of pregnancy related complications (primarily due to placental dysfunction) in EPB group, such as placental abruption, preterm labour, IUGR, PPROM, LBW, CS are – Weiss et al 200414, Patel et al 200015, Alcazar et al 200016, Das et al 199617.Increased risk of placental abruption and IUGR could not be documented in present studies, but was reported by Mulik et al 200418, Szekeres- Bartho et al 200219.Double the risk of preterm delivery has been reported by William et al 199120, Batzofin et al 198421 in EPB group but was found to be mildly raised in our study (7.6%).Although the incidence of stillbirths in EPB group was not significant in present study as contrary to other studies such as – Batzofin et al 198421, Funderburk et al 198022. Perinatal outcome observed during this study supports the evidence in literature that pregnancy bleeding (irrespective of GA) is an important and independent predictor of adverse perinatal outcome. The present study suggests that the greater risk to pregnancies affected by vaginal bleeding is premature delivery before 37 weeks (36%). This factor alone may be responsible for other findings including LBW, depressed one and five minute APGAR scores and increased perinatal mortality. Other studies are – Savita et al 20085 (41.8%), Fauzia et al 20104 (79.16%), Martha et al23 (71%), Cotton et al 198024 (77.5%). Many other studies have correlated threatened miscarriages with preterm delivery – Weiss et al 200414, Mulik et al 200418, Williams et al 199120.Babies delivered as IUD and stillbirths in LPB group were found to be 5.9% and 3%. ( Fauzia et al 20104 reported 42.1% fresh stillborns in APH bleeding ).Babies with IUGR and those requiring NICU admission in LPB group were 14.7% and 22% respectively. 2.9% had neonatal deaths ( early and late) and rest were discharged alive (88.2%). Other studies reporting perinatal mortality in APB cases are – Fauzia et al 20104 (50.2%), Savita et al 20085 (23.7%), Arora et al 200125 (61.5%), Khosla et al 198926 (53.5%).The same complications were not significantly raised in the EPB group due to small sample size. Outcome of pregnancies affected by EPB ended in spontaneous abortion in 30.3% of cases, 7% were ectopic and 3% were molar pregnancy. The rest 34% cases progressed to viable pregnancy but major and minor complications involved. Newborns of LPB cases suffered from a variety of complications of pretem delivery and perinatal asphyxia. No significance association was observed between prevalence of congenital anomalies in either group, as also in other studies(Batzofin et al 198421, Funderburk et al 198022). With previous threatened miscarriages, pregnancy may be more complicated with preterm delivery, PPROM, lower birth weight.27-31Study conducted by Sipila et al suggests that first and second trimester vaginal bleeding are important predictors of adverse neonatal outcomes. Both first and second trimester bleeding were associated with high fetal loss.32 Bleeding prolonged into the second trimester is likely to affect fetal outcome adversely. That limited to the first trimester may either end in abortion or (if not severe) may not affect fetal health unduly as enough recovery time is available.33,34 Study conducted by Evrenos et al indicates that women who have vaginal bleeding in the first trimester are at increased risks of later pregnancy complications; especially preterm delivery, shortened mean pregnancy period, lower gestational fetal weight and preterm rupture of membrane.27,28,35 Bleeding during first trimester was associated with increased risk of preterm delivery.29 Because of impaired implantation and invasive trophoblasts, spontaneous abortion may occur in early pregnancy while preterm delivery, PPROM, placental abruption and preeclampsia may happen in later period.28-30 In a study conducted by Hossain et al preterm delivery and PPROM rates were increased in the threatened miscarriage group.18,29-31,36,37 Lower Apgar scores after 1 and 5 minutes were expected in threatened abortion group because of increased rates of preterm delivery.37 Strobinoand Silverman have linked frequency of low birth weight and preterm birth with the severity of maternal hemorrhage.38 The prognosis of abruptio placentae complications depends on whether treatment is received by the patient, on the quality of treatment, and on the severity of the abruption. Outcomes for the baby also depend on the gestational age. The prognosis on the fetus is worse, currently, in the UK, about 15% of fetuses die following this event. Without any form of medical intervention, as often happens in many parts of the world, placental abruption has a high neonatal mortality rate.39 The baby may be born as a low birth weight. The fetus may be deprived of oxygen and thus suffer from asphyxia. Placental abruption may also result in fetal death, or stillbirth. The newborn infant may have learning issues at later development stages, or even requiring professional aid.40Thus, APH remains important because it is relatively frequent with potential adverse outcomes for the mother and the neonate. The high neonatal mortality and morbidity seen with APH is mostly due to its strong association with preterm delivery.41-43Series of studies have demonstrated that APH is an independent factor for adverse neonatal outcomes in very preterm, as well as late preterm neonates. Among these groups APH has been independently associated with increased neonatal mortality and morbidity, particularly severe respiratory disorders, increased admission rate to NICU and increased hospital stay.44-47 In a study conducted by Johns J et al both preterm delivery and PPROM are related with low birth weight as predictable factors. Our study demonstrated that the fetal weight was lower in the case than control group. It is related with births at earlier gestations.37,48 In a study conducted by Yang J, Bleeding in both trimesters was associated with preterm birth due to preterm labor (RR = 3.6, 95% CI: 1.9, 6.8). Bleeding of multiple episodes, on multiple days, and with more total blood loss was associated with an approximate twofold increased risk of earlier preterm birth, PPROM, and preterm labor. In contrast, bleeding in the second trimester only, of a single episode, on a single day, and with less total blood loss was not associated with any category of preterm birth.49 Recurrent bleeding predicted preterm birth more strongly than did single episodes, consistent with previous studies, as stated by Williams et al.50,51 However, a series of studies have demonstrated that APH is an independent factor for adverse neonatal outcomes in very preterm, as well as late preterm neonates. Among these groups APH has been independently associated with increased neonatal mortality and morbidity, particularly severe respiratory disorders, increased admission rate to NICU and increased hospital stay.52-55Some forms of placental dysfunction causes vaginal bleeding in late pregnancy (Alexander et al 2000) and are well established as causes of preterm birth (Tuzovic et al 2003). Including pregnancies complicated by placental abnormalities could account in part for the reported association between vaginal bleeding in the aggregate and preterm birth.54,55 Further insights regarding the association between vaginal bleeding and preterm birth are likely to come only from more detailed understanding of the underlying causes of the bleeding or more refined and complete assessment of bleeding characteristics. Beyond statistical prediction of preterm birth, the biologic and clinical meaning of this common pregnancy complication remains to be fully elucidated.49
CONCLUSION Patient who had vaginal bleeding of any amount, in pregnancy, should be monitored vigilantly. Preparations should be made for premature delivery and measures taken to arrest premature labor as needed. Obstetrician should ensure that the patient delivers in a well- equipped centre where intrapartum and neonatal complications can be recognized and managed with neonatologists. Early recognition of risk factors for early and late pregnancy bleeding is essential. Whether occurring in early or later half of pregnancy, bleeding has significant impact on perinatal outcome. Pregnancy bleeding (irrespective of gestational age), is an independent predictor of adverse perinatal outcome.
REFERENCES
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home