|
Table of Content - Volume 16 Issue 3 - December 2020
Comparison between use of oral misoprostol versus vaginal misoprostol for induction of labour at term
O Balajojamma
Associate Professor, Department of OBG and Gynaecologist, Ayaan Institute of Medical Science, Ranga Reddy dist, INDIA. Email: balaonteddu@gmail.com
Abstract Background: In modern obstetrics, around 30% of cases require induction of labour for various reasons. Misoprostol is gaining popularity as pharmacological inducing agent, though the route and dosage of administration are not standardised. The objective of the study is to compare the safety and efficacy of the two routes of misoprostol administration—oral (50 μg 4th hourly) and vaginal (25 μg 4th hourly), for induction of labour at term Methods : In this randomised trial, 100 women having crossed the expected date of delivery without going into spontaneous labour and cases which had premature rupture of membranes <12 h were considered for labour induction and were divided into two equal groups. Group A received 50 μg misoprostol orally 4th hourly, and group B received 25 μg misoprostol vaginally 4th hourly. Labour characteristics and maternal and foetal outcome were compared. Results: In terms of maternal outcome, mean number of doses for oral group is 2.73 and vaginal group is 3.04. In oral group, mean induction to vaginal delivery interval was 13 h 43 min and in vaginal group interval is 13 h 26 min which was statistically not significant. The need for oxytocin augmentation was also statistically not significant. Both groups had equal number of failed inductions. Emergency LSCS done for foetal distress was more in vaginal group 2.9% compared to oral group which is 1%, but difference was not statistically significant (p value −0.55). Number of thick MSL in oral group was 3.2% as compared to vaginal group which is 10.7% which was statistically significant (p value −0.04). APGAR score at 5 min 7/10 was seen in 7.7% in vaginal group as compared to 0% in oral group which was also statistically significant (0.004). Number of NICU admissions was also more in vaginal group compared to oral group. Keywords: induction of labour, misoprostol, fetal outcome, maternal outcome
INTRODUCTION Induction of labour is a well-established obstetric concept since ancient times. Induction of labour is one of the most common procedures in obstetrics. In modern obstetrics, induction is indicated when benefits to either the mother and/or the foetus outweigh the risks in continuing the pregnancy. It could be elective or emergency induction of labour. In modern times, 10–33% obstetric cases require induction of labour. Induction of labour is defined as iatrogenic stimulation of uterine contraction to accomplish delivery prior to the onset of spontaneous labour aimed at delivery by vaginal route1,2.There are various methods of induction of labour falling in two broad categories: non-pharmacological and pharmacological. Amongst various methods used for ripening of cervix and induction of labour, prostaglandin E1 (misoprostol) is safe, reliable, cheap, easily applicable, and readily available, which results in good maternal and foetal outcome. Prostaglandin E1 (misoprostol) tablets as an inducing agent of labour by various routes, e.g. vaginal, oral, and rectal, have received huge attention. The American College of Obstetricians and Gynaecologists (2000, 2003) has reaffirmed the use of misoprostol as a drug for induction of labour because of its proven safety and efficacy. This ‘Off label’ use of misoprostol has gained acceptance amongst international bodies of obstetricians. The ideal dose and route of administration are, however, still subject of debate.
MATERIALS AND METHODS Demographic details such as age, height, weight, parity, gestational age and indication for induction, and AFI are noted. Bishop’s score before induction is assessed by per vaginal examination. After obtaining informed consent, they will be randomised to receive either 50 μg of oral or 25 μg of vaginal misoprostol. Before administration of drug, each woman will have a pelvic examination to assess the Bishop’s score to rule out active labour. The dose will be repeated every 4th hourly for a maximum of six doses for both groups A and B. The dose will be with held in the presence of active labour, ≥3 contractions over 10 min or a cervical dilation of ≥4 cm. From the time of induction of labour to delivery, the patients were closely monitored for signs of labour, progress of labour, uterine contractions, and FHR monitored by intermittent auscultations. If the patient went into the active phase of labour, artificial rupture of membrane was done if required. In case of failure of induction, the patient was taken for LSCS directly. A total of 100 women at term with indication of labour induction admitted in AYAAN hospital over a period of 1 year will be included in the study, from August 2019 to july2020. Patients who receive 50 μg oral misoprostol will be considered as group A and those who receive 25 μg vaginal misoprostol will be considered as group B. Inclusion CriteriaLive singleton pregnancy of gestational age of 37–42 weeks. Nulliparous women. A cephalic presentation. Postdated pregnancy. Premature rupture of membranes. Bishop score <6. Exclusion CriteriaPrevious uterine scar. CPD. Antepartum haemorrhage. Multiparity. Multiple gestation. Oligohydraminos. Polyhydraminos..IUGR. Medical disorders like diabetes mellitus and hypertension. Contraindication to prostaglandins like asthma. Preterm premature rupture of membranes. History of glaucoma and epilepsy. METHOD OF DATA COLLECTION A total of 100 women at term with indication of labour induction admitted in Ayaan hospital over a period of 1 year will be included in the study, . It is a prospective randomised study. The patients were divided into two groups.
RESULTSData were entered in Excel format and analysed using EPI INFO software. Percentage and frequencies were calculated for categorical data, and Chi-square test was done to know association between groups A and B. Mean and standard deviations were calculated for continuous variables, and Student’s t test was done to find association between two groups. p value ≤0.05 at 95% confidence interval was considered significant. Majority of the women belonged to age group 21–25 years. Numbers of women in gestational age between 37 and 40 weeks are 41.3% out of which 19.2% belong to oral group and 22.1% belong to vaginal group. A total of 58.7% of women were above 40 weeks of gestation, out of which 30.8% of women belonged to oral group and 27.9% belonged to vaginal group. Indications for induction in both the groups were postdated and premature rupture of membranes. For oral group, the mean pre-induction score was 2.98. For vaginal group, the mean pre-induction score was 2.52).
Figure 1: Distribution of number of doses Mean number of doses for oral group is 2.73, and vaginal group is 3.04 with p value of 0.227
Figure 2: Distribution of mode of delivery For oral group, 43.3% (45 cases) proceeded for normal delivery, 3.8% (4 cases) required LSCS intervention, and 2.9% (3 cases) required vacuum application for delivery. For vaginal group, 41.3% (43 cases) proceeded for normal delivery, 6.7% (7 cases) required LSCS intervention, 1% (1 case) required forceps application for delivery, and 1% (1 case) required vacuum application for delivery.
Table 1
In oral group, mean induction to vaginal delivery interval was 13 h 43 min. Minimum induction to vaginal delivery interval was 5 h 15 min. Maximum induction to vaginal delivery interval was 26 h 15 min. In vaginal group, mean induction to vaginal delivery interval was 13 h 26 min. Minimum induction to vaginal delivery interval was 4 h 16 min ). Figure 2: Distribution of colour of liquor
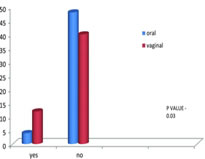
For oral group, 41.3% (43 cases) exhibited clear liquor, 5.8% (6 cases) exhibited thin MSAF, and 2.9% (3 cases) exhibited thick MSAF. For vaginal group, 35.6% (37 cases) exhibited clear liquor, 4.8% (5 cases) exhibited thin MSAF, and 9.6% (10 cases) exhibited thick MSAF. In oral group, there were three cases of thick MSL, and when compared to vaginal group, there were 10 cases. p value −0.04 was significant. In oral group, out of 3 cases, 1 delivered normally, 1 case underwent vacuum-assisted delivery, and 1 case underwent LSCS. In vaginal group, out of 10 cases, 5 delivered normally, 1 case by vacuum-assisted delivery, 1 case by forceps, and 3 cases underwent LSCS. For oral group, 1% (1 case) had nausea and vomiting and uterine hyperstimulation. A total of 3.8% (4 cases) had nausea and vomiting. In total, 1% (1 case) of mothers experienced uterine hyperstimulation For vaginal group, 2.9% (3 cases) mothers developed fever 2.9% (3 cases) of mothers experienced uterine hyperstimulation, and p value is 0.14 which shows no significant difference. Distribution of indication for LSCSFor oral group, 3.9% (4 cases) required emergency LSCS. Of the 4 cases, 3 cases were taken for LSCS due to failed induction. In total, 1 case was taken for LSCS due to thick meconium. For vaginal group, 6.9% (7 cases) required emergency LSCS. Of the 7 cases, 1 case was taken for LSCS due to DTA, 3 cases was taken for LSCS due to foetal distress, and 3 cases were taken for LSCS for failed induction. For oral group, mean 1 min score was 7.40. Mean 5 min score was 8.520. Six neonates (5.8% cases) had APGAR score <6 at 1 min. None of neonates had APGAR score <6 at 5 min. For vaginal group, mean 1 min score was 7.21. Mean 5 min score was 8.42. Two neonates (1.9% cases) had APGAR score <6 at 1 min. None of the neonates had APGAR <6 at 5 min. A total of 7.7% of cases (8 cases) had APGAR 7 at 5 min (p value −0.004)
Figure 5: Distribution of NICU admissions DISCUSSIONIn terms of maternal outcome, mean number of doses for oral group is 2.73 and vaginal group is 3.04. In another study done by Khatri et al.3 found that there was no difference in mean number of doses required for both oral and vaginal groups. In oral group, mean induction to vaginal delivery interval was 13 h 43 min and in vaginal group interval is 13 h 26 min which was statistically not significant. The need for oxytocin augmentation in oral group was 15, and in vaginal group it was 12; however, this difference was also statistically not significant. In another study done by Khatri et al.3 showed that although interval was earlier in oral group than vaginal group (group A −15.5 and group B 15.03 h, respectively), it was statistically insignificant showing that both routes are equally efficacious. In another study done by Rasheed et al.4 showed that the mean induction to delivery interval was significantly shorter in the vaginal group compared with the oral group (13.5 vs. 20.6 h, p < 0.010). In a study done by Emmanuel et al.5 showed that the vaginal route reduced the mean induction to delivery interval by four and half hours (20.7 ± 12.1 vs. 16.2 ± 10.4; mean difference 4.50, 95% CI 0.63–0.82; p = 0.02). Both groups had equal number of failed inductions. Emergency LSCS done for foetal distress was more in vaginal group 2.9% compared to oral group which is 1% but difference was not statistically significant (p value −0.55). According to study done by Komala et al.6, 94% of cases delivered vaginally in oral group and in vaginal group 86% of cases delivered. Numbers of cases with fever and hyperstimulation were more with vaginal group, and nausea and vomiting were more in oral group although this difference is not statistically significant. Number of thick MSL in oral group was 3.2% as compared to vaginal group which is 10.7% which was statistically significant (p value −0.04). APGAR score at 5 min 7/10 was seen in 7.7% in vaginal group as compared to 0% in oral group which was also statistically significant (0.004). Numbers of NICU admissions were also more in vaginal group compared to oral group. This finding is consistent with previous studies done by Uludag et al.7; this was 16.7% cases in vaginal and 5.9%. Of cases in oral group had meconium-stained liquor. A study done by Khatri et al.3 (compared 100 μg oral and 25 μg vaginal) showed similar results. In studies done by Komala et al.6 and Deshmukh et al.8, no difference was seen between both groups. Abbassi et al.9 showed oral route was better with respect to treatment interval, and number of doses required. CONCLUSIONMisoprostol in either oral or vaginal route has proven to be equally effective for inducing labour in women at term pregnancy. However, occurrence of lesser incidence of meconium-stained liquor and NICU admissions and fewer caesareans with better neonatal outcome in women induced with oral misoprostol outweighs its advantages over the vaginal misoprostol. However, further studies are required to standardise the dosage for oral route of misoprostol for induction of labour. This study showed that oral route of misoprostol is equally efficacious as vaginal route with less neonatal complications making it more safe. Both authors declare that they have no conflict of interest. Human and Animal Right Statements All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with Helsinki Declaration 1975, as revised in 2008. Ethical statement This study was approved by the institutional ethical committee. Informed Consent Informed consent was obtained from all patients for being included in the study. REFERENCES
Policy for Articles with Open Access:
|
|
||||||||||||||||
 Home
Home