|
Table of Content - Volume 17 Issue 2 - February 2021
Study of thyroid stimulating hormone (TSH) in pregnancy in Telangana population
Vijayalakshmi Chirumamilla
Associate Professor, Department of Obstetrics and Gynaecology, Mediciti Institute of Medical SciencesGhanpur Medchal – 501401. Telangana, INDIA. Email: drvijayalakshmi05@gmail.com
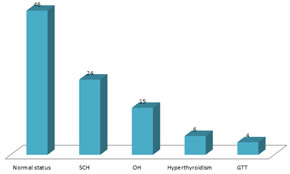
Abstract Background: Thyroid dysfunction is commonly encountered in pregnancy and can affect maternal and foet al. outcomes. Method: 95 pregnant women were evaluated clinically. TSH, free T4, TPO antibody test were carried out. TSH, free T4 were estimated by chemilumiscence using immulite 100 TSH assay was used with normal range of 0-4-4.0 ml U/L/ the lower detection limit was 0.004mlU/L. Normal Thyroid values were followed by the guide lines of American Thyroid association. Results: 46 (48.4%0 had normal thyroid status, 24 (25.2%0 had SCH 15 (15.7%) OH, 6 (6.3%) Hyper thyroidism. 4 (4.20%) GTT. The clinical manifest station were – Dry skin was in 2 (8.3%) in SCH, 3 (20%) in OH. Cold intolerance was in 3 (12.5%) in SCH, 4 (2.66%) in OH, puffiness – 2 (8.3%) in SCH, 2 (13.3%) in OH, Parasthesis 2 (13.3%) in OH, 2 (33.3%) in Hyperthyroidism weight loss in 2 (33.3%0 in Hyperthyroidism, constipation 4 (16.6%) in SCH, 3 (20%) in OH, Goitre 6 (13%) in Normal, 5 (20.8%) in SCH, Hyper emesis 1 (2.5%) in GTT Conclusion: This TSH study in pregnant will be helpful to obstetric and Gynaecologist, Endocrinologist to treat such patients efficiently and prevent various risk for foetus and maternal health. Keywords: TSH, Free T4, GTT, SCH, OH, Hyperthyroidism.
INTRODUCTION It is well documented that maternal thyroid dysfunction is associated with adverse outcomes in the mother and foetus, including miscarriage, preterm delivery, eclampsia, pre-eclampsia and placental abruption.1,2 Decreased availability of maternal thyroid hormone may also impair neurological development of foetus including SCH, OH, GTT (3). On the other hand hyperthyroidism also at the increased risk for spontaneous miscarriage if un-treated, moreover congestive heart failure, thyroid storm, preterm birth, foet al. growth restriction, increased perinatal morbidity and mortality.4 Overt Hypothyroidism leads to endemic iodine are often associated with hypothyroxinemia. Untreated OH in Pregnancy has increased risk for premature birth low birth weight and miscarriage.5 Hence it was mandatory to evaluate the thyroid dysfunction during pregnancy for normal growth of foetus and safeguard the health of mother too.
MATERIAL AND METHOD 95 Pregnant women regularly visiting obstetrics and Gynaecology department of Mediciti Institute of Medical Sciences Ghanpur Medchal-501401 Telangana were studied. Inclusive Criteria: Pregnant women irrespective of gestation age having symptoms of thyroid hormones abnormalities were selected for study. Exclusion Criteria: The pregnant women already on thyroid treatment (medication), history of Diabetes mellitus, hypertension or any chronic illness were excluded from the study. Method: Clinical evaluation was done to look for features thyroid dysfunction including goitre. The gestational age and expected delivery date was noted in every patient 2 ml blood was collected and analysed for TSH, free T4 and anti TPO antibody (Thyroid peroxide antibodies) in their first visit. TSH and free T4 was planned to be repeated at necessary intervals TSH and free T4 were estimated by chemiluminiscene using immolate 1000. TSH assay was used with normal range of 0.4 – 4.00 ml U/L. The lower detection limit was 0.0004 ml U/L. The intra assay coefficient of variation was 4.5% and inter assay coefficient of variation was 8% Free T4 was estimated by immulite 1000 with a normal range of 0.89 – 1.76 mg/dL. The intra assay coefficient variation was 4.5% and inter assay coefficient of variation was 8% Ant TPO antibody was done by chum luminescence method (immulite 1000). The lower detection limit was 7 IU/mL. Anti TPO> 150 IU/mL was taken as positive. As per guidelines of American Thyroid association 2011
Hyperthyroidism was defined as suppressed TSH<0.1 ml U/L with normal or elevated fT4.
The duration of study was about 2 years (July-2018 to August-2020) Statistical analysis: Various findings were classified with percentage. The analysis was carried out in SPSS software. This research paper was approved by ethical committee of Mediciti Institute of Medical Sciences Ghanpur Medchal-501401, Telengana.
OBSERVATION AND RESULTS Table-1: Normal laboratory profile of Thyroid Hormone. Table-2: Normal value of TSH in each trimester Table-3: Study of thyroid hormone in pregnant women 46 (48.4%) were normal, 24 (25.2%) had SCH, 15 (15.7%) had OH, 6 (6.31%) had hyperthyroidism, 4 (4.21%) had GTT. Table-4: Clinical manifestations in pregnant women (a) Dry skin – 2 (8.3%) in SCH, 3 (20%) in OH, (b) cold intolerance – 3 (12.5%) In SCH, 2 (13.3%) in OH, (c) Puffiness – 2 (8.3%) in SCH, 2 (13.3%) in OH (d) Parasthesis – 2 (13.3%) in OH and 2 (33.3%) in Hyperthyroidism (e) Weight loss – 2 (33.3%) in Hyperthyroidism (f) Constipation – 4 (16.6%) in SCH, 3 (20%) in OH (g) Goitre – 6 (13%) in Normal, 5 (20.8%) in SCH, (h) Hyper emesis – 1 (25%) in GTT Hyperthyroidism.
Table 1: Normal laboratory ranges of Thyroid assay
Graph 1: Normal laboratory ranges of Thyroid assay
Table 2: Normal values in each trimester
Table 3: Status of thyroid Hormone in pregnant women
SCH= Subclinical Hypothyroidism, OH= Over Hypothyroidism, GTT= Gestational Thyrotoxis
Table 3: Status of thyroid Hormone in pregnant women
Table 4: Clinical Manifestations in Thyroid dysfunction (No. of patients: 95)
DISCUSSION In the present study of TSH in pregnancy in Telangana Population. The normal status women were 46 (48.4%), 24 (25.2%) SCH, 15 (15.7%) OH, 6 (6.31%) Hyperthyroidism, 4 (4.21%) GTT (Table-3). The clinical manifestation were Dry-skin was observed in 2 (8.3%) in SCH, 3 (20%) in OH, cold intolerance, 3 (12.5%) in SCH, 4 (26.6%) in OH, Puffiness 2 (13.3%) in OH, 2 (33.3%) in Hyperthyroidism, parenthesis 2 (13.3%) in OH, 2 (33.3%) in Hyperthyroidism weight loss 2 (3.33%0 in Hyperthyroidism, Constipation 6 (13%) in Normal, 5 (20.8%), 3 (20%) in OH, 2 (33.3%) in Hyperthyroidism, 1 (25%) Hyper emesis in GTT (Table-4). These findings were more or less in agreement with previous studies. There are two common causes of Hyperthyroidism in pregnancy are to Grave Disease (GD), due to thyroid stimulation by TRAbs and GTT. GTT influence elevated human chorionic gonadotrophin (HCG) levels during pregnancy which causes thyrotoxis symptoms (9) while GD causes sleeplessness, anxiety fatigue, weight loss, presence of goitre, ocular changes10. Auto immune thyroditis remains the most common cause for both hypothyroidism and Hyperthyroidism. WHO recommends 200 micrograms of daily iodine intake by mother. The foet al. thyroid gland begins to produce thyroid hormone at about 10 to 12 weeks of gestation, so during first trimester, foetus depends on maternal supply of thyroid hormones which is necessary for foet al. growth. From second trimester onwards demand is met by mother and foetus11. The availability of thyroxin to the developing foet al. neuron is vital for their maturation and proper function. Maternal alteration in thyroid function, OH or SCH, can adversely affect the foetus directly by way of trans placental passage of abnormal level of maternal hormone, thyroid antibodies or prescribed medication or indirectly by way of altered physiology. Serum T3 and T4 levels rise 30 minutes after delivery and persist for 5 days. This is due to TSH elevation caused by stress delivery. So new born screening should be done from the cord blood or 5 days after delivery.12
SUMMARY AND CONCLUSION Although screening of thyroid dysfunction is not yet for recommendation, it should be considered for the prediction of un-wanted consequences. TSH level study is sufficient and should be done pre conceptionally or early in first trimester. But this study demands further hormonal, genetic, patho-physiological study because there are no exact parameters to find out the quantum of hormones.
REFERENCES
Policy for Articles with Open Access
|
|
 Home
Home