|
Table of Content - Volume 21 Issue 3 - March 2022
Assessment of oxidative and anioxidants status in pregnant woman- An intuitional study
K Madhuri1, Naimisha Movva2*, Koratala Anoop3
1,2Associate Professor, 3Resident, Department of Obstetrics and Gynaecology, Mamata Medical College And General Hospital, Khammam, Telangana, INDIA. Email: mamatakhmm@gmail.com
Abstract Background: Oxidative stress is characterised as an imbalance between reactive oxygen species generation and antioxidant defence capacity. Numerous disorders have been linked to the pathophysiology of oxidative stress in pregnancy. The aim of this study was to understand oxidative stress induced free radicals and role of antioxidant capacity in pregnant women. Material and methods: The present study included 75 patients who have been admitted in the Department of Obstetrics and Gynaecology, with normal pregnancy. Parameters of the oxidative status and antioxidant capacity in serum and whole blood were evaluated pregnant women. Results: the present study showed, the mean age was 22.65 years with a mean gestational age of 36.75 weeks. The reactive species like NO RNI has shown higher levels in the serum of the entire patient pregnant woman. Whereas, there was no significant difference in the antioxidants tested (GST, GSH, SOD, GPx) when compared to control group. TBARS levels showed significant elevation in pregnant woman when compared to control group. Conclusion: This study shows that changes in free radical and antioxidant defences occur as a result of the body and circulation alterations that occur during pregnancy. Keywords: Antioxidants; free radicals, pregnancy; Malonyldialdehyde.
INTRODUCTION Pregnancy is known to be associated with changes in the physiological and metabolic functions of a woman's life. Pregnancy is well-known to extend the oxidative stress, a phenomenon generated by a traditional systemic inflammatory response, which ends up in high amounts of circulating reactive oxygen species (ROS) leading to potential tissue damage.1 Oxidative stress occurs when there is a loss of balance between the production of the reactive species in the living organisms and the antioxidant power regulated by both antioxidant enzymes and the antioxidants involved.2 Antioxidants are substances capable of curtailing either the initiation or propagation of injury induced by free radicals. Enzymatic antioxidants include Superoxide dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX) which are endogenous enzymes. Vitamin C and Vitamin E belong to non-enzymatic antioxidants which are obtained from exogenous dietary sources.3Because of the increased metabolic requirement, pregnancy might cause an oxidative imbalance. Pregnancy issues resulting from oxidative imbalance can include Eclampsia, miscarriages, etc.4 Because pregnancy necessitates a high level of energy expenditure, the growth of the maternal antioxidant system to battle any potential problems requires special attention. According to previous studies, alteration in antioxidant defence mechanisms and, increased metabolic demand causes increased generation of reactive oxygen species during normal uncomplicated pregnancies.5Although the release of free radicals is a normal physiological activity, an excess of them can act on lipids and induce lipid peroxidation. Antioxidants prevent free radical-induced tissue damage by preventing the formation of scavenging radicals or by promoting their decomposition.6 The first line of defence against ROS consists of antioxidant enzymes such as SOD, glutathione peroxidase (GPx), and catalase (CAT) which metabolize these reactive species to innocuous by-products.7Oxidative stress can be measured in three major ways: the direct measurement of ROS levels; the indirect measurement of protein, lipid, and DNA damage instead of assessing oxidative stress; the assessment of antioxidant status, which can be an indirect method for measuring oxidative stress. This present study was to evaluate the oxidative stress and antioxidant mechanisms in pregnant women.
MATERIAL AND METHODS The present study included 75 patients who have been admitted in the Department of Obstetrics and Gynaecology, Mamata Medical College, Khammam. A written informed consent from all the patients and Ethical Committee approval was obtained before starting the study. A detailed history, general physical examination and obstetric examination were performed. Pregnant women with the following conditions were excluded from the study: Bleeding disorders, Women on non-steroidal anti-inflammatory drugs such as aspirin, Splenomegaly, Connective tissue disease such as SLE, Hypertension, HIV and hepatitis B infection. Information such as drug history, presence of splenomegaly and HIV / hepatitis B status were extracted from the clinical notes. Specimen: Blood sample (random) was taken from each pregnant woman, serum sample was obtained by centrifugation of blood samples at 2000rpm for 10min, and it was stored at −20◦ until the date of analysis. Serum nitric oxide was measured in terms of its products, nitrite and nitrate, by the method of Griess modified by Fiddler.8 GSH was estimated in plasma by the method of Thomas and Skrinska,9 which is based on the reaction of GSH with 5,5′, dithiobisnitrobenzoic acid to form a complex that absorbs at 412 nm. The results were expressed as mg/dl plasma. The estimation of MDA in plasma was done by the method of Draper and Hadley.10 The color produced by the reaction of thiobarbituric acid with MDA was measured colorimetrically at 533 nm. The results were expressed as nmoles/ml plasma Statistical analysis: Data analysis was done by using SPSS Package version. Simple proportions, mean, standard deviation and Student “t” test and Chi-square test was used to find out the association between two groups. P value of less than 0.05 is considered as statistically significant.
RESULTS Table 1: Comparison of characteristics and clinical features in between normal healthy individuals and patients groups.
The general characteristics of the pregnant women participating in the study are sown in Table 1. In this study, the mean age of the study participants were 22.65 ±2.47 years. Most of the study subject’s parity was with mean of 2.07. The gestational age of the woman was 36.75± 2.53 weeks. (Table 1) Table 2: Estimation of Nitric Oxide (NO) levels (µmol/dl) and Reactive Nitrogen Intermediates (RNI) levels ((nmol/ml) in the control and patient groups.
The concentration of total NO was higher in the serum of all the patient pregnant woman. The statistically significant differences were observed in the total NO concentrations in the serum between the patients with the control group. Similarly, there was a significant rise in the Reactive Nitrogen Intermediates (RNI) levels in the patients group compared to the control group (Table 2). The increase in the patients group was significantly more than the control group. Data represent by Mean ± SE, *p < 0.05, significant **p < 0.01, very significant. Table 3: Estimation of antioxidant levels in the control and patient groups
Data represent by Mean ± SE, The all tested Biochemical parameters (GST, GSH, SOD, GPx) showed no significantly alteration towards the baseline levels of tested antioxidants. The patients in all the trimester did not showed any significant decrease in the all tested parameters when compared with the respective control groups (Table 3).
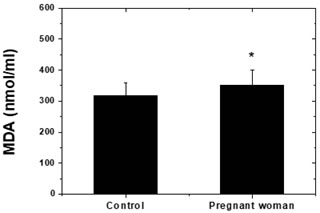
Figure 1: Estimation of Malonyldialdehyde (MDA) levels (nmol/ml) in the control and patient group. Control subjects did not result in lipid peroxidation. Pregnant woman showed significant elevation of lipid peroxidation products (TBARS) observed when compared to control group (Figure 1). Data represent by Mean ± SE, Significant *p < 0.05.
DISCUSSION The present study demonstrates the oxidative stress and antioxidant mechanisms in pregnant women. Pregnancy is a physiological state characterised by a high metabolic demand and increased tissue oxygen requirements,11 which results in a rise in reactive oxygen species generation.12 Monitoring of the oxidative stress in pregnant women is important to enable an understanding of the relationship between oxidative stress and pregnancy outcome.13Oxygen-derived free radicals participate in redox reactions leading to oxidative modifications in biomolecules, among which proteins and lipids are preferential targets. Pregnancy is a natural occurrence. The initial phases of development in a normal pregnancy take place in a low oxygen environment called physiological hypoxia of the early gestational sac, which is helpful because it protects the developing foetus from the harmful and teratogenic effects of throughout all phases of pregnancy, excessive ROS stimulation can result in hyperglycemia, IUGR, miscarriage, and spontaneous abortion. Placental oxidative stress can be induced by a variety of reasons, including maternal history, genetics, and environmental factors, and it can result in poor pregnancy outcomes.14 In the present study, statistically significant differences were observed in the concentration of total NO and Reactive Nitrogen Intermediates levels were higher in the serum of all pregnant woman. The negative impacts of reactive species during pregnancy can affect the pregnancy's progress, the foetus' development, and the infant's condition upon birth. So there is always need for the defence mechanism in the form of antioxidants which play an important role in scavenging activity.15 Antioxidants are substances or molecules that are capable of reducing or stopping the effects of oxidants. These substances include, among others, micronutrients like some vitamins and trace elements, some metallo-enzymes like catalase, superoxide dismutase and glutathione peroxidase.5 In normal pregnancy, superoxide dismutase levels have been found to rise. Superoxide dismutase and glutathione peroxidase are the most prominent natural antioxidant enzymes that can eliminate ROS. SOD activity in the blood begins to rise during the first months of pregnancy. SOD protects embryos from lipid peroxidation, which is especially important in the early stages of pregnancy. In women with preeclampsia, serum SOD activity is lower than in normal pregnant women. In placental homogenization, the SOD level is low in the early stages of pregnancy but may increase by 2-3 times with the progression of pregnancy. The reason for the change in placental SOD levels during gestational period is due to the change in placental oxygen requirement during pregnancy. The placental SOD activity in placenta-related pathologies was lower than in normal pregnant women.16 In the study performed by Jenkins,17 serum SOD activity in preeclampsia groups was found to be statistically significantly higher compared to the control group. However, the literature also reported that SOD activity in preeclampsia was higher, lower and no significant differences than control group. Superoxide dismutase (SOD) and glutathione peroxidase (GPx) are the most prominent natural antioxidant enzymes that can eliminate ROS. In the present study, antioxidant enzyme (SOD, GST, GPx) activities were increased compared to control patients. The results of the present study clearly showed that circulating levels of thiobarbituric acid-reactive substances is significantly decreased in women with normal pregnancy and levels were marked as the gestation period increases. Simiar observation was seen in earlier studies.18 To conclude, oxidative stress in the form of ROS is the primary contributing factor in a variety of pregnancy complications. Although the antioxidant defence has been designed to limit the creation of ROS, the increased amount of ROS cannot be managed, resulting in oxidative stress. To reduce the prevalence of reproductive disorders, future research should focus on optimising the breakdown of intracellular ROS and increasing antioxidant bioavailability.
REFERENCE
Policy for Articles with Open Access
|
|
 Home
Home