|
Table of Content - Volume 19 Issue 1 - July 2021
U C Jha1, Rishabh Yadav2*, Ashutosh Kumar3
1Associate Professor and H.O.D, 2,3Junior Resident, Department of Medicine, Darbhanga Medical College and Hospital, Bihar, INDIA. Email: rishabhmgims@gmail.com
Abstract Background: Hypertension is one of the most common complex disorders. The etiology of hypertension differs widely amongst individuals within a large population. And by definition, essential hypertension has no identifiable cause. However, several risk factors have been identified. Aim: To determine the effect of Calcium Channel Blocker (Efonidipine) on Heart rate and Blood pressure in patients with mild-to-moderate hypertension. Methods: It is an observational, prospective study using convenient single random sampling, who were mild-to-moderate hypertension Study subject included 100 Primary Hypertensive Patients attending Department of General Medicine in Darbhanga Medical College and Hospital, During the study period November 2018 to October 2019. Results: The comparison of heart rate, trough Sitting SBP/Sitting DBP, and 24-hour ambulatory mean SBP/DBP from baseline to week 4 among not well controlled patients revealed that the above-mentioned variables are not significantly reduced at 4th week follow up compared to baseline (p value=>0.05). In these patients the dosage of efonidipine was increased up to 60 mg once daily. Conclusion: Efonidipine can be safely used to decrease BP with a reduction in HR in patients with mild-to-moderate essential hypertension. These results suggest that efonidipine may be therapeutically useful as an initial agent for essential hypertension.
INTRODUCTION Essential hypertension also called primary and idiopathic hypertension is the form of arterial hypertension that by definition has no identifiable cause. It tends to be multi-factorial. and is likely to be the consequence of an interaction between environmental and genetic factors. Prevalence of essential hypertension increases with age, and individuals with relatively high blood pressure at younger ages are at increased risk for the subsequent development of hypertension. The JNC-8 classification recommends blood pressure criteria for defining normal blood pressure, hypertension (stages I and II), and isolated systolic hypertension, which is a common occurrence among the elderly. Hypertension is considered to be present when a person's systolic blood pressure is consistently = or>140 mmHg and/or diastolic blood pressure is = or >90 mmHg.1 Efonidipine hydrochloride, a new generation dihydropyridine (DHP) calcium channel blocker, inhibits both T-type and L-type calcium channels.2 Typical DHP calcium antagonists, such as nifedipine and amlodipine, selectively inhibit L-type calcium channels of vascular smooth muscle cells, resulting in vasodilation. In addition to exhibiting an antihypertensive effect through vasodilation by blocking L-type calcium channels, efonidipine hydrochloride has also been suggested to regulate heart rate (HR) by inhibiting the T-type calcium channels, which are localized primarily in the sinoatrial node and are involved in the pacemaker mechanism of the heart.3 Furthermore, efonidipine has been reported to have a negative chronotropic effect, which may be involved in controlling tachycardia.4 Working on sinoatrial node cells by inhibiting T-type calcium channel activation, efonidipine prolongs the late phase-4 depolarization of the sinoatrial node action potential, which suppresses an elevated HR. Previous studies have shown that efonidipine decreased the HR in contrast with other DHPs in patients with essential hypertension.5 Recent epidemiologic studies have confirmed earlier studies that showed resting HR to be an independent predictor of cardiovascular and all-cause mortality, independent of gender or pre-existing cardiovascular disease.[6] Clinical trial results also suggest that reduction in HR alone is an important benefit afforded by beta-blockers in patients with angina pectoris, acute myocardial infarction, and chronic heart failure. Regarding hypertension treatment, an approved consensus guideline has recommended antihypertensive medication with HR-lowering properties, especially in females and the elderly.[7] The aim of this study was to determine the effect of efonidipine on HR and blood pressure (BP) in patients with mild-to-moderate essential hypertension.
METHODS Type of Study: It is an observational, prospective study. Study Design: It is an observational, prospective study using convenient single random sampling, who were mild-to-moderate hypertension (sitting diastolic BP 90-110 mmHg). After a 2-week washout, eligible patients were treated with Efonidipine (40 mg once daily for 12 weeks). The primary end point was change in HR from baseline to week 12. The secondary end-point included the change in trough sitting BP and 24-hour mean BP between baseline and week 12. Laboratory and clinical adverse events were monitored at each study visit (4, 8, and 12 weeks). Place of Study: Department of General Medicine, Darbhanga Medical College and Hospital, Bihar. Sample Size: Study subject included 100 Primary Hypertensive Patients attending Department of General Medicine in Darbhanga Medical College and Hospital. Duration of Study: November 2018 to October 2019 Inclusion Criteria:
Exclusion Criteria:
Methodology: The present study was conducted after obtaining clearance and approval from the Institutional Ethics Committee Darbhanga Medical College and Hospital, Bihar, written informed consents was taken from the patients. This study was conducted in the Department of General Medicine and it was an observational prospective study. The study was conducted with duration from November 2018 to October 2019. A total of 100 patients presented with mild to moderate essential hypertension who attended the OPD Medicine were consecutively recruited. Demographic data was collected under the following headings: age, sex, anthropometric parameters. Weight and height were measured while patients were dressed in light clothing. Body mass index was calculated as weight (kg) divided by the square of the height (m). After the 2-week washout period of previous antihypertensive medications, patients were given 40 mg efonidipine at 8:00 a.m. (±2 hours). If the SiSBP was >140 mmHg or the SiDBP was >90 mmHg at 4 or 8 weeks, the dosage of efonidipine was increased up to 60 mg once daily. Clinical follow-up was performed at 4, 8, and 12 weeks of treatment. At each visit, the SiSBP, SiDBP, and pulse rate were measured. The resting HR was measured manually for 1 minute after the patient had rested in a sitting position for 30 minutes. The BP was measured as follows: the same time of day, before dosing, the same arm, by the same investigator. Ambulatory BP monitoring (ABPM) was performed twice at baseline and at the end of the 12-week treatment. At each visit, patients were asked about adverse events (AEs). The primary efficacy variable was the mean change in rest-ing HR from baseline to week 12. The secondary efficacy variables included the change from baseline of the mean office tr-ough BP (i.e., 24 hours after the last dosing) and the 24-hour mean BP assessed with ABPM after 12 weeks. The response rate was defined as the proportion of patients in which the SiDBP was <90 mmHg or had decreased by ≥10 mmHg from baseline after 12 weeks of treatment. Tolerability was assessed based on the incidence of AEs. Medical history was updated and laboratory tests were performed at each study visit. All laboratory and clinical AEs were assessed by the investigators in terms of their relationship to the study drug and intensity. Serious AEs were defined as those that were life-threatening, required hospitalization, or were associated with significant permanent disability or congenital malformation. Statistical Analysis: Data was checked for accuracy and completeness then coded and entered into (Statistical Package for the Social Sciences) version 19.0 for analysis. The results presented in frequency tables, cross tabulations and figures. Categorical data are presented as frequency with percentages. Continuous data with normal distribution are presented as mean with standard deviation. The difference of the baseline characteristics and change in heart rate and BP before and after medication was tested using a Unpaired t-test. and One way ANOVA, p value <0.05 was considered statistically significant.
RESULTS Table 1: Age Distribution
Age distribution of the study subjects is presented in Table 1. Highest occurrence was observed in 51-60 years age group that is 38% followed by 28% in 41-50 years age group and 15% in 31-40 years age group, with a mean age of 48.5 years.
Table 2: Sex Distribution
Table 2 presents the distribution according to gender. From 100 patients, 63 patients (63%) were male and 37 (37%) were female. Table 3: Previous use of Anti-hypertensive
Table 3 shows the incidence of previous use of anti-hypertensive. 11% of the study subjects were previously using antihypertensive.
Table 4: Levels of heart rate, trough Sitting SBP/Sitting DBP, and 24-hour ambulatory mean SBP/DBP at baseline
Table 5: Changes in heart rate, trough Sitting SBP/Sitting DBP, and 24-hour ambulatory mean SBP/DBP from baseline to week 4 among well controlled patients (n=79)
Table 5 shows the comparison of heart rate, trough Sitting SBP/Sitting DBP, and 24-hour ambulatory mean SBP/DBP from baseline to week 4 among well controlled patients. It shows the above mentioned variables are significantly reduced at 4th week follow up compared to baseline in well controlled patients (p value=<0.05).
Table 6: Changes in heart rate, trough Sitting SBP/Sitting DBP, and 24-hour ambulatory mean SBP/DBP from baseline to week 4 among not well controlled patients (n=21)
Table 6 shows the comparison of heart rate, trough Sitting SBP/Sitting DBP, and 24-hour ambulatory mean SBP/DBP from baseline to week 4 among not well controlled patients. It shows the above-mentioned variables are not significantly reduced at 4th week follow up compared to baseline (p value=>0.05). In these patients the dosage of efonidipine was increased up to 60 mg once daily.
Table 7: Changes in heart rate, trough SiSBP, SiDBP and 24-hour ambulatory mean SBP, DBP from baseline to week 12
Table 7 shows the comparison of heart rate, trough Sitting SBP/Sitting DBP, and 24-hour ambulatory mean SBP/DBP from baseline to week 4, week 8 and week 12 among all patients. It shows the above-mentioned variables are significantly reduced at 4th, 8th and 12th week follow up compared to baseline in all patients (p value=<0.05). 3 patients were not available for the final follow up at week 12.
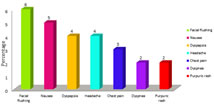
Incidence of Adverse Events Figure 1: Incidence of Adverse Events
Incidence of adverse events among study subjects is presented in figure. The most common AE was facial flushing (6 patients), followed by nausea (5 patients), dyspepsia (4 patients), headaches (4 patients), chest pain (3 patients), dyspnea (2 patients), and a purpuric rash (2 patients).
DISCUSSION Hypertension is one of the most important risk factors for cardiovascular diseases, including ischemic and haemorrhagic stroke, dementia, ischemic heart disease, heart failure, vision loss, and kidney failure. Hypertension is a multifactorial and multifaceted disease in which elevated blood pressure is only one sign of multiple underlying physiological abnormalities.8 Hypertension or high blood pressure is a leading cause of death. The condition is often called a “silent killer” because its symptoms can go undetected until damage to the body has occurred. Because of this, it is one of the most significantly under-diagnosed and under-treated medical conditions all over the world. High blood pressure is usually a lifelong condition. High blood pressure can occur at any age but is particularly prevalent in people with a family history of high blood pressure, people who are overweight or obese, people with diabetes, and heavy drinkers.9 Efonidipine hydrochloride, a new generation dihydropyridine (DHP) calcium channel blocker, inhibits both T-type and L-type calcium channels.[3] Because efonidipine hydrochloride inhibits both L- and T-type Ca channels, it is called a dual Ca channel blocker. Typical DHP calcium antagonists, such as nifedipine and amlodipine, selectively inhibit L- type calcium channels of vascular smooth muscle cells, resulting in vasodilation. In addition to exhibiting an antihypertensive effect through vasodilation by blocking L-type calcium channels, efonidipine hydrochloride has also been suggested to regulate heart rate (HR) by inhibiting the T-type calcium channels, which are localized primarily in the sinoatrial node and are involved in the pacemaker mechanism of the heart.3 Furthermore, efonidipine has been reported to have a negative chronotropic effect, which may be involved in controlling tachycardia.4orking on sinoatrial node cells by inhibiting T-type calcium channel activation, efonidipine prolongs the late phase-4 depolarization of the sinoatrial node action potential, which suppresses an elevated HR. Previous studies have shown that efonidipine decreased the HR in contrast with other DHPs in patients with essential hypertension.5 Tanaka et al.2 showed that efonidipine decreases the HR and plasma aldosterone more than amlodipine in patients with hypertension. Furthermore, this negative chronotropic effect has been suggested to exert an anti-arrhythmic action. Kinoshita et al.10 demonstrated that efonidipine reduces arrhythmias and sudden death in a mouse model of dilated cardiomyopathy by repolarizing the resting membrane potential and normalizing the cardiac autonomic nervous system imbalance. These findings suggest potential therapeutic applications for efonidipine as an antihypertensive agent for patients at high risk for fatal arrhythmias and sudden death, such as patients with severe he-art failure. Currently, the role of beta-blockers as first-line antihypertensives has been challenged.8 A meta-analysis by Lindholm et al.11 suggested that the risk of stroke was 16% higher with beta-blockers than with other drugs and the risk of all-cause mortality was 3% higher. In fact, beta-blockers were excluded from routine initial therapy for hypertension in the 2006 British Hypertension Society guidelines. However, the HR-lowering effect of beta-blockers is still required in certain hypertensive patients, such as those with high sympathetic activity. Our findings suggest that efonidipine might be a better alternative to beta-blockers as an initial antihypertensive medication. In this study, once daily efonidipine was effective not only in the control of mild-to-moderate hypertension, but also in the reduction of HR, which is significantly associated with clinical outcome in hypertension.[12] Finally, a 12-week course of treatment with efonidipine was well-tolerated and no treatment-related serious AEs occurred.
CONCLUSION Efonidipine can be safely used to decrease BP with a reduction in HR in patients with mild-to-moderate essential hypertension. These results suggest that efonidipine may be therapeutically useful as an initial agent for essential hypertension. The present study suggests that efonidipine hydrochloride decreases the HR and exhibits favorable effects on the autonomic nervous system. This action may be very significant, as high HR and increased sympathetic nervous activity are important cardiovascular risks or factors influencing prognosis in patients with hypertension.
REFERENCES
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home