|
Table of Content - Volume 20 Issue 3 - December 2021
Cardiac autonomic neuropathy in type 1 diabetes
Thahsin Taikadan1, Soopy Kayanaduth2*, Basheer M P3
1Senior Resident, Department of General Medicine, Government Medical College, Manjeri, Kerala, INDIA. 2Associate Professor, Department of General Medicine, Government Medical College, Kozhikode, Kerala, INDIA. 3Professor, Department of Physiology, Al Azhar Medical College, Thodupuzha, Kerala, India. Pin: 685605, INDIA. Email: drsoopy@gmail.com
Abstract Background: Cardiac Autonomic Neuropathy (CAN) is one of the major risk factors behind cardiovascular mortality among people with Type1 Diabetes. Early diagnosis of cardiac autonomic neuropathy using simple tests helps for the prevention of these complications. Ewing et al. described 5 simple tests to assess CAN. The objective of the study was to estimate the prevalence of cardiac autonomic neuropathy among 75 patients with Type1 diabetes using Ewing tests and to predict the risk factors associated with CAN. Methodology: We conducted a hospital based cross sectional study. The study included 75 patients with Type1 diabetes of more than 5 years duration. Type1 diabetes patients with age more than 18 years attending outpatient units under the department of general medicine Government medical college, Kozhikode during the study period of January 2018 to January 2019 was taken as study population. All patients were subjected to detailed history and physical examination. Ewing tests were used to detect the CAN. Tests were done using physiopac software at the physiology department of government medical college Kozhikode. Ewing Tests included Heart rate response to deep breathing, heart rate response to Valsalva maneuver (Valsalva ratio), blood pressure response to standing, diastolic blood pressure response to handgrip and heart rate response to standing (30:15ratio). Data was analysed using Microsoft excel and SPSS software. Results: The prevalence of CAN according to Ewing tests among 75 patients with Type1 DM was 60%. The heart rate response to deep breathing was abnormal among 80% of patients. Heart rate response to standing was abnormal in 44%. The prevalence of early CAN was 40% and the prevalence of severe CAN was 20%. There was significant association between cardiac autonomic neuropathy and poor glycemic control, disease duration, resting heart rate, triglyceride level and microvascular complications like retinopathy, neuropathy and nephropathy. There was no significant association between cardiac autonomic neuropathy age, sex, BMI, systolic and diastolic blood pressure. Conclusions: CAN is a serious chronic complication of diabetes and it is an independent predictor of cardiovascular mortality. Assessment of CAN using simple non-invasive tests like Ewing can be used for its early diagnosis and thereby help to reduce the adverse outcomes. The prevalence of CAN was 60% in our study. The most important risk factors for CAN identified in this study was duration of diabetes, poor glycemic control, elevated triglyceride level and resting heart rate. The microvascular complications of type1 diabetes were significantly associated with CAN. Furthermore, a good glycemic control can delay the development of CAN. Keywords: Type 1 diabetes cardiac autonomic neuropathy; Ewings tests.
INTRODUCTION Diabetes mellitus (DM), is fast-gaining the status of a global pandemic. In the absence of proper interventions, the number of people afflicted by the disease is projected to reach 629 million globally and 151 million in Southeast Asia by 2045. India has the second largest diabetic population (72.9 million) after China (114.4 million), and is expected to surpass China by 2045 (India: 134.3 million; China: 119.8 million). Many studies in India have shown a prevalence rate as high as 13% in some rural and urban areas.1 The metabolic dysregulation associated with diabetes mellitus leads to secondary pathophysiologic changes in multiple organ systems, which are associated with high morbidity and impose a tremendous burden on the health care system, if they are not treated timely and adequately. Among these, cardiovascular disease is one of the most common complications that increase mortality in these patients. The cardiovascular complications can be divided into Atherosclerotic coronary artery disease, Diabetic cardiomyopathy and Cardiac autonomic neuropathy.2 Diabetic Autonomic Neuropathy (DAN) is among the least recognized and understood complications of diabetes, despite its significant negative impact on survival and quality of life in people with diabetes.3 It is also a major source of increased cost in caring for the diabetic patient. The metabolic disorders of diabetes lead to diffuse and widespread damage of peripheral and autonomic nerves, and small vessels. When diabetic neuropathy affects the autonomic nervous system, it can damage the cardiovascular, gastrointestinal, genitourinary and neurovascular systems, and impair metabolic functions such as glucose counter‐regulation. Of these, Cardiac Autonomic Neuropathy (CAN) encompasses damage to the autonomic nerve fibers that innervate the heart and blood vessels, resulting in abnormalities in heart rate control and vascular dynamics. CAN is a significant cause of morbidity and mortality associated with a high risk of cardiac arrhythmias and sudden death. CAN is associated with a poor prognosis and result in severe orthostasis, postural hypotension, exercise intolerance, enhanced intraoperative instability, and an increased incidence of silent myocardial infarction (MI) and ischemia.4 Now it is possible to objectively identify early stages of CAN with the use of careful measurement of autonomic function and to provide therapeutic choices that are based on symptom control and that might abrogate the underlying disorder. CAN is an early and frequent but under diagnosed complication of diabetes mellitus (DM) affecting from 7 to 15% of newly diagnosed patients to 90% of those in line for double transplant. Its clinical manifestations include resting tachycardia, exercise intolerance, intraoperative and perioperative cardiovascular instability, orthostatic hypotension, orthostatic tachycardia, bradycardia syndromes, silent myocardial infarction, cardiac denervation syndrome, and sudden death. CAN is strongly associated with approximately five-fold increased risk of cardiovascular mortality. CAN is further classified to no cardiac autonomic neuropathy, early cardiac autonomic neuropathy and severe cardiac autonomic neuropathy.5 CAN in type1 diabetes is less studied among Indian population. The tests needed to identify CAN are simple and cost effective. In a developing country like India early identification of CAN can significantly reduce morbidity and mortality and also helps for better treatment and preventive strategies. In this work we studied the prevalence and risk factors associated with CAN in Type 1 diabetes.
METHODOLOGY This study was conducted at the outpatient clinic of the Department of Medicine, Government. Medical College, Kozhikode, Kerala, India and was approved by the Institutional Ethics committee of Government Medical College, Kozhikode (Ref. No. GMCKKD/RP 2018/IEC/16). A total of 75 patients with type 1 diabetes (n = 75), who attended the outpatient clinic of the Department of Medicine from January 2018 to January 2019 were included in this Cross-sectional study. Inclusion criteria: Patients with >18 years and with Type 1 diabetes of >5 years duration. Exclusion criteria: Patients with congestive cardiac failure, ischemic heart disease, chronic alcoholism, untreated thyroid disorders, anaemia (Hb <12), CKD grade 4 or 5, pregnancy, patients on beta blockers, diuretics, anti-depressants, anti-arrhythmic, alpha agonist or alpha blockers were excluded from the study group. Experimental Design: After getting informed consent from the patients, proper history would be taken and followed by a detailed clinical examination. ECG leads will be connected and continuous monitoring will be done using physiopac software in physiolab. Heart rate will be calculated at various phases like inspiration, Valsalva and immediate standing. Blood pressure will be measured at 3rd minute of standing. Hand grip BP by grip dynamometer with 30% of maximum power. Then, the patients would be subjected to the following 5 tests6
The participant in lying position will be asked to breathe quietly and deeply at six breaths a minute (five seconds in and five seconds out) for one minute. An electrocardiogram will be recorded continuously throughout the period of deep breathing with time noted separately to indicate the onset of each inspiration and expiration using 16 channel psycho physiopac. The maximum and minimum R-R intervals during each breathing cycle will be measured and converted to beats per minute. The result is then expressed as the mean of the difference between maximum and minimum heart rates for the six measured cycles, in beats per minute. A difference of 15 beats or more will be considered to be normal, mean values between 11 to 14 beats per minute as borderline, and values of 10 beats or less per minute as abnormal.
With the participant lying quietly on the bed the heart rate will be recorded continuously on an electrocardiograph. The participant will be then asked to stand up unaided and the point of starting to stand is marked on the electrocardiogram. The shortest R-R intervals at or around the 15th beat and the longest R-R interval at or around the 30th beat after starting to stand will be measured, the heart rate response will be expressed as the 30:15 ratio. Values of 1.04 and above is normal, those between 1.01 and 1.03 is borderline and values of 1.00 and less is considered abnormal.
The patient is asked to blow in to the rubber tubing of a sphygmomanometer and hold it at a pressure 40mm of mercury for 15 seconds while continuous ECG was recorded. Then patient will be asked to breathe normally with continuous ECG monitoring. The manoeuvre will be performed thrice with a one-minute interval in between them. The result will be expressed as the ratio of longest R-R interval after the manoeuvre and to the shortest RR interval during the manoeuvre.
Blood pressure will be recorded twice. First in lying posture and after three minutes of standing. The difference in systolic pressure is calculated.
The maximum voluntary contraction of hand is measured using grip dynamometry initially and the hand grip test is done by maintaining the grip at 30% of the maximum up to 3 minutes using dynamometer. The result is expresses as the difference between highest diastolic blood pressure during the handgrip and the normal diastolic blood pressure before the hand grip. The results of each of the above 5 tests are classified in to 3 separate groups based on the severity of abnormality detected, and each of them is given a definite point as described by Bellavere et al.,7
Table 1: CAN Score (Bellavere et al.,)
Table 2: Categorization of patients based on the CAN score
DATA ANALYSIS Data assessment was done by using SPSS software and Microsoft excel. The frequency and percentage relation between the prevalence of cardiac autonomic neuropathy was assessed via SPSS. The association between Cardiac autonomic neuropathy and various clinical indicators have been demonstrated with appropriate statistical models like chi square test.
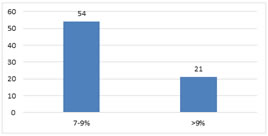
RESULTS Gender and Age distribution of Subjects Males contributes about 54 (72%) and females contributes about 21(28%) of the total 75 study subjects. Minimum age among 75 participants were 18 and the maximum age was 38. The mean age was 21.6 years. The major age group was among 20-30 years. HbA1C The mean HbA1C of study population was 8.79% (Fig. 1). 72% of the study population (54 out of 75) were having HbA1C between 7 to 9%. Remaining 28% were having HbA1C >9%. Figure 1: HbA1C
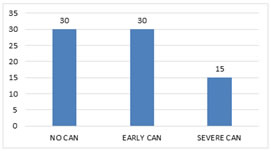
Duration of Type1 diabetes The mean duration of Type1 diabetes among the subjects were 10.4 years. 60% (45) were having duration of less than 10 years. Whereas 40% (30) of the subjects were having duration of more than 10 years. Longest duration among study subjects was 28 years. Cardiac Autonomic Neuropathy The prevalence of cardiac autonomic neuropathy among 75 patients was 60% (Fig. 2). Out of 75 subjects 30 patients were having early CAN that is 40% with a CAN score between 2 - 4. 15 patients, that is 20%, were having severe CAN with a CAN score of 5.
Figure 2: CAN Prevalence
Peripheral Neuropathy Out of 75 patients, 39 patients (52%) had peripheral neuropathy which was diagnosed clinically and 36 patients (48%) didn’t have peripheral neuropathy. Diabetic Retinopathy Among 75 patients, 48% that is 36 had diabetic retinopathy. 52% that is 39 subjects didn’t have diabetic retinopathy. Diabetic Nephropathy Among 75 patients 48 that is 64% didn’t have diabetic nephropathy, whereas 36% were having nephropathy defined by urine Albumin creatinine ratio more than 30mg/g. HbA1C and CAN There was significant association between cardiac autonomic neuropathy and the value of HbA1C with a p value of 0.005 (Table 3).
Table 3: CAN and HbA1C
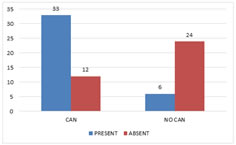
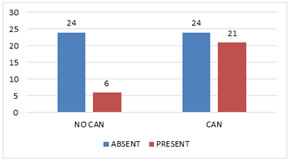
CAN and Peripheral Neuropathy There was significant association between CAN and peripheral neuropathy with a p value of <0.001 (Fig 3).
Figure 3: Cardiac Autonomic Neuropathy and Peripheral Neuropathy
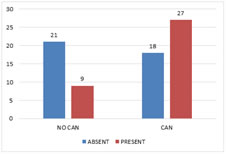
CAN and Retinopathy There was a significant association between cardiac autonomic neuropathy and retinopathy with a P value of 0.011 (Fig 4). Figure 4: Cardiac Autonomic Neuropathy and Retinopathy
CAN and Nephropathy CAN and nephropathy also showed a significant association with a P value of 0.018 (Fig 5). Figure 5: Cardiac Autonomic Neuropathy and Nephropathy
BMI and CAN The mean BMI was 24.2. There was no significant association between BMI and CAN.
Table 4: BMI and CAN
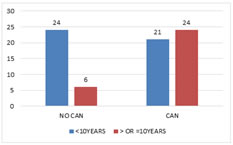
Duration and CAN The mean duration of diabetes was 10.04 Years. There was significant association between duration of diabetes and incidence of cardiac autonomic neuropathy with a P value of 0.004 (Fig 6). Figure 6: Cardiac Autonomic Neuropathy and Duration Resting Heart Rate and CAN The mean heart rate among 15 patients without CAN was 71.60. The mean heart rate among patients with CAN was 84.9. There was significant association between CAN and resting heart rate with a p value of <0.001 (Table 5).
Table 5: Resting Heart Rate and CAN
Systolic Blood Pressure and CAN The mean systolic blood pressure (SBP) among those with cardiac autonomic neuropathy was 121.53 and those without autonomic neuropathy was 124.37. The p value was 0.264. There was no significant association between systolic blood pressure and cardiac autonomic neuropathy (Table 6). Table 6: Systolic Blood Pressure and CAN
Diastolic blood pressure and CAN The mean diastolic blood pressure (DBP) among those with CAN was 76.33 and among those without autonomic neuropathy was 73.03. After doing unpaired t test the P value was 0.141. There is no significant association between diastolic blood pressure and CAN in our study subjects (Table 7).
Table 7: Diastolic Blood Pressure and CAN
Fasting Blood Sugar and CAN The mean fasting blood sugar (FBS) value among 45 subjects with CAN was 206.33. After doing unpaired t test the p value was <0.001. It shows a significant association between fasting blood sugar values and cardiac autonomic neuropathy (Table 8).
Table 8: Fasting Blood Sugar and CAN
Triglycerides and CAN The mean value of triglycerides among patients with CAN was 219. After doing unpaired t test the p value was <0.001 indicating significant association between triglyceride level and CAN (Table 9).
Table 9: Triglycerides and Cardiac autonomic neuropathy
Cardiac Autonomic Function Tests The most common abnormal test was heart rate variability. Heart rate variability was present among 80% of patients with cardiac autonomic neuropathy. The next common abnormality was in 30:15 ratio test with a prevalence of 44%. Abnormal diastolic blood pressure response to handgrip was noted only in 3% (Table 10).
Table 10: Cardiac Autonomic Function Tests
DISCUSSION In our study cardiac autonomic neuropathy was assessed by cardiac autonomic reflex tests proposed by Ewing et al., in 1980. Diabetes affects parasympathetic fibers and sympathetic fibers independently. Hence the tests should be conducted independently for diagnosing sympathetic and parasympathetic deficits. Ewing’s criteria use the tests for parasympathetic as well as sympathetic component but the sympathetic component is used only for diagnosis of severe CAN.6 Prevalence of CAN Prevalence of CAN, based on assessment of abnormal cardiovascular autonomic function tests, is found to be varying in different studies. This may be because each study uses different study population, and different methods for CAN detection. At a tertiary referral centre the prevalence of CAN in diabetics can be as high as 80%. The prevalence of cardiac autonomic neuropathy in our study was 60%. Early cardiac autonomic neuropathy was present in 40% of the subjects. Severe CAN was present in 20% of the subjects. Anjum et al., (2017) conducted a study in a tertiary care centre in India among patients with type1 and type 2 Diabetes mellitus. The prevalence of cardiac autonomic neuropathy was 57.1% among patients with type1 diabetes comparable to the present study. The prevalence of early CAN was 4.1% and definite CAN was present among 53.1%8 Guo et al., (2019) conducted a study in China with 73 patients showed a CAN prevalence of 61.6%.9 Whereas a study conducted in Korea by Kim et al., (2019) showed a CAN prevalence of 12.6%.10 According to a study conducted by Spallone et al., (2011) fifty-five patients (31.4%) out of the 175 included did not have CAN at laboratory assessment. Sixty patients (34.2%) had possible CAN, 43 (24.6%) had a confirmed CAN, and 17 (9.7%) had severe CAN.11 The study conducted by Pappachan et al., (2008) in a tertiary care centre in Kerala with 100 subjects with type1 and type 2 diabetes mellitus showed a 60% prevalence of cardiac autonomic neuropathy.12 In a study conducted by Kempler et al., (2002) among randomly selected Type I (insulin‐dependent) diabetic patients from 31 centres in 16 European countries, the prevalence of autonomic neuropathy was 36% with no sex difference.13 The prevalence can range from 2.5% (based on the primary prevention cohort in the Diabetes Control and Complications Trial) to as high as 90% of patients with type 1 diabetes.14 CAN and Blood Sugar Levels In our study there was significant association between cardiac autonomic neuropathy and HbA1c values with a p value of 0.005. Those with CAN had higher HbA1c when compared to those without cardiac autonomic neuropathy. The diabetes causes hyperglycaemia and it is intuitive to accept that sustained hyperglycaemia as estimated by HbA1c should correlate with occurrence of diabetic complications like autonomic dysfunction. Long term prospective studies have shown that mean HbA1c over time correlates with severity of the autonomic dysfunction.15 There was significant association between fasting blood sugar values and cardiac autonomic neuropathy with a P value of 0.001. The mean FBS was 206.33 in those with cardiac autonomic neuropathy indicating a poor glycemic control. A study by Moţăţăianu et al., (2018) also showed that, there was significant association between cardiac autonomic neuropathy and duration, glomerular filtration rate, peripheral neuropathy and HbA1c.16 CAN and Microvascular Complications In our study peripheral neuropathy was present in 39 patients that is 52%. Retinopathy was present in 48%. Diabetic nephropathy was present in 36%. There was significant association between diabetic neuropathy, nephropathy and retinopathy and CAN with p values 0.001, 0.018 and 0.011 respectively. In a study conducted by Moţăţăianu et al., (2018) among 34 patients with type1 diabetes, the prevalence of peripheral neuropathy and retinopathy was 64% and 58% respectively.16 Pappachan et al., (2008) also reported that there was significant association between cardiac autonomic neuropathy and microvascular complications like peripheral neuropathy.12 These finding suggests that clinicians should pay more attention to patients with diabetic retinopathy or nephropathy over and above the strict glycemic control required for the prevention of CAN. CAN and Duration of Diabetes The mean duration of diabetes in our study was 10.04 years. There was significant association between cardiac autonomic neuropathy and duration of diabetes with a P value <0.001. The longer the duration of diabetes, the more likely is the occurrence of hyperglycaemic states even in adequately controlled diabetic. CAN and Other Parameters There was a significant association between resting heart rate and CAN with a p value of 0.001. The mean heart rate among those with CAN was 84.93. High resting heart rate among patients with CAN indicates increase in sympathetic tone secondary to vagal tone impairment.17 The mean fasting triglyceride level in those with cardiac autonomic neuropathy was 219.27. There was significant association between cardiac autonomic neuropathy and fasting triglycerides with a p value of <0.001. In a study conducted by Jaiswal et al., (2018) among adolescents and young adults with type1 and type 2 diabetes, poor long term glycemic control (HbA1c), high blood pressure and elevated triglycerides correlated with cardiac autonomic neuropathy.18 There was no significant association between cardiac autonomic neuropathy and BMI, systolic blood pressure and diastolic blood pressure. The autonomic scores did not correlate significantly either with the age of the patient or with gender. The age of patient at which the diabetes develops and the age at which it is diagnosed is variable. An elderly patient may have diabetes of few months duration while young age patient may have diabetes of few years’ duration. Most of the patients in this study was between the ages of 20 to 30 years. Duration of disease rather than the age of patient is the independent risk factor for the development of autonomic dysfunction. Among Ewing tests, the most common abnormal test was heart rate variability. Heart rate variability was present among 80% of cardiac autonomic neuropathy patients. The next common abnormality was in 30:15 ratio test with a prevalence of 44%. The least noted abnormal test was diastolic blood pressure response to handgrip. In the study conducted by Pathak et al., (2017) cardiac autonomic function tests of HR variability during deep breathing, Valsalva maneuver ratio, HR response to standing, BP response to standing, and BP response to sustained handgrip found abnormal response in 68%, 40%, 52%, 12%, and 14%, respectively.19
CONCLUSION AND RECOMMENDATIONS In this study the prevalence of Cardiac Autonomic Neuropathy in patients with type1 diabetes with a disease duration of more than 5 years with age more than 18 years was found to be 60%. The prevalence of CAN was significantly associated with duration of diabetes, HbA1c, fasting blood sugar value and triglycerides. The occurrence of CAN was also significantly associated with diabetic microvascular complications. BMI and blood pressure didn't show a significant association with cardiac autonomic neuropathy. However, resting heart rate has got significant association with cardiac autonomic neuropathy. Presence of CAN has been correlated with increase in morbidity and mortality. CAN increases the risk of intraoperative and peri-operative hemodynamic instability, intra-operative hypothermia, development of orthostatic hypotension and silent myocardial infarction. Given the high incidence of diabetes and high prevalence of CAN, it is recommended that testing for CAN should be done as a routine work-up of diabetic patients. The procedures for performing tests for detection of CAN are safe, non-invasive and require minimal infrastructure.
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home