|
Table of Content - Volume 19 Issue 3 - September 2021
Visual evoked potentials (VEP) studies in primary open glaucoma
Bhagya V1, Jyothi V2*, Shanshad Begum3
{1Professor, Department of Physiology} {3Professor, Department of Psychiatry} JJM Medical College and Hospital, Davanagere, Karnataka, INDIA. 2Assistant Professor, Department of Ophthalmology, SSIMS and RC, Davanagere, Karnataka, INDIA. Email: dnandanajyothi2009@gmail.com
Abstract Background: With increasing prevalance of primary open angle glaucoma and optic nerve damage as a consequence of this, there is a need for early diagnosis and prevention of optic nerve damage. VEP, the potential recorded from the occipital region in response to the visual stimuli can be used for early detection of the primary open angle glaucoma. Visual evoked potential (VEP) is a non invasive method to assess the visual pathway. The present study was done to evaluate the impact of primary open angle glaucoma on central nervous system particularly, visual pathway. Methods: 30 primary open angle glaucoma patients attending outpatient department of ophthalmology department, SS hospital, Davanagere and 30 age matched controls selected randomly from general population were subjected to Visual evoked potential. Parameters for VEP such as latencies of waves N70, P100, and N155 peak-to-peak amplitudes of waves N70-P100 and P100-N155 were assessed and analyzed by using unpaired student-T test for comparison between cases and controls. Results: The present study observed that the there was a statistically significant increase in P100 latency in cases compared to the controls. And also there was a statistically significant decrease in N70 and P100 amplitude in cases compared to the controls. Conclusion: The present study correlates with earlier findings that visual pathway gets involved in primary open angle glaucoma even before the development of neuropathy which can be detected using VEP. Meticulous follow-up is a must to prevent the complications of primary open angle glaucoma, so that further damage can be prevented. Keywords: Primary Open Angle Glaucoma; Vision; Visual Evoked Potential (VEP);
INTRODUCTION Primary open angle glaucoma with an IOP more than 21 mm Hg at some point in the course of the disease, is generally a bilateral but not always symmetrical disease characterized by adult onset, associated with an open angle of normal appearance, glaucomatous optic nerve head damage, and visual field loss1. One of the common eye disease, characterized by optic neuropathy, and often associated with elevated intraocular pressure, leading to characteristic visual field defects and optic nerve head damage. It is well known that damage to the ganglion cells and/or their axons produces these visual field defects. but, it is less clear the extent to which the ganglion cells undergo a rapid apoptotic death as opposed to lingering in an abnormal state. If the latter fact holds, then it raises the possibility of neuroprotection of unhealthy retinal ganglion cells response2. The visual evoked potential (VEPs) which assess the integrity of the neural pathways for vision, is a useful electrophysiological indicator of early visual changes in primary open glaucoma. The optic nerve joins retina with the brain. The receptors or end organs through which the visual impulses are mediated are rods and cones of retina. They are stimulated by the light impulses and synapse with the inner nuclear or bipolar layers; the cells of which in turn synapse with the ganglion cell layer. The axons of ganglion cell form optic nerve. The optic nerve extends from the retina to the optic chiasma. Approximately 1 million fibers of optic nerve are unmyelinated in the retina and optic nerve head; but these become myelinated as they pass through lamina cribrosa. The P100 waveform of VEP is generated in the striate and peristriate occipital cortex not only due to activation of primary cortex but also due to volleys. On giving pattern or flash stimulation, along with increased f19 show a increase in metabolic activities.4 Visual evoked potential (VEP) is the recording of electrical changes that can be recorded from the scalp in response to a repetitive light stimulation of the eyes.3Electrophysiologic recording of visual evoked potential has been very useful in evaluating visual function.4VEP stimulation can be used in diagnosis of glaucoma and also used for the assessment of optic nerve diseases5. VEP latency can be used as a measure of early glaucomatous damage before retinal ganglion cell death occurs2. Hence, it is used as a marker of reversible ganglion cell damage. Visual evoked potentials (VEPs) assess the integrity of the visual pathways from the optic nerve to the occipital cortex. Optic disc cupping and visual field loss are associated with prolongation of VEP latency. Therefore, the current study is done to find the effect of glaucomatous damage on VEP latencies and amplitudes. Evoked potentials are easier and non invasive tool for evaluation of central nervous system.6 Recording of visual evoked potentials using pattern reversal stimuli is a very sensitive test for detecting any anterior visual pathway abnormalities .7 VEP is primarily a reflection of activity in the central 3-6 degrees of visual field, that is relayed to the surface of occipital lobe. The projections that arise from the peripheral retina are directed to the regions deep within the calcarine fissure.8 The VEP can be used for detecting anterior visual conduction disturbance; however, it is not specific in terms of etiology. It is very useful in evaluating visual function. It is noninvasive and has excellent temporal resolution. Patients with primary open angle glaucoma have subclinical visual defect as revealed by impaired Visual Evoked Potential. In this study primary open angle glaucoma patients showed delayed latencies and reduced amplitude of P100. Glaucoma is characterized by progressive loss of retinalganglion cells along with their axons.9 The electrical pulses from the ganglion cells are transmitted to the cerebral cortex via the optic nerve, optic tract, lateral geniculate nucleus, and theoptic radiations. VEP can be used to monitor any interruption of this transmission of these electrical pulses.10 It has been shown that up to 40% of the total nerve fibres at the nerve head may be lost before any significant changes are found on perimetry. And also pathological cupping represents advanced nerve damage. To detect primary open angle glaucomaat an early stage, a number of advanced automated perimetric techniques have been developed,11but they are limited by their subjectivity, especially in elderly patients.In recent years studies of visually evoked potentials (VEP) have been used in order to identify and monitor nerve fibre damage.12 Pattern reversal VEP is more reliable than flash VEP in clinical diagnosis of primary open angle glaucoma (Aminoff).2
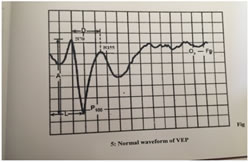
MATERIALS AND METHODS The study was conducted in the department of Physiology, J.J.M. medical college, Davanagere. In this study, 30 patients with primary open glaucoma between 25 to 55years referred from ophthalmologists of various hospitals in and around Davangere were selected and 30 normal age matched subjects were selected randomly from the general population. Recording was carried out in a quiet and dimly lit room. Subjects were asked to come without applying oil to scalp and to shampoo hair and make it dry. VEPs were recorded using the PC based, 4 channel, RMS EMG Salus machine manufactured by RMS RECORDERS and MEDICARE SYSTEM, Chandigarh and standard silver- silver chloride disc electrodes. A VEP monitor displaying checker board was used to give the pattern reversal stimulus. A montage consisting of one channel was used for the VEP recording. The subject was asked to sit comfortably in front of the checkerboard pattern at an eye screen distance of 100cm. An amplification which ranged between 20,000 and 1,00,000 was used to record the VEPs. The electrode impedance was kept below 5KΩ. The recording was performed in a dark and sound attenuated room. Uni-ocular stimulation was given to both eyes separately with black and white checks which changed phase (black to white and white to black) abruptly and repeatedly at a specified number of reversals per second, by using a checker board. The usual glasses (if any) were allowed to be put on during the test. The subject was instructed to avoid the usage of meiotic or mydriatic drugs, 12 hours before the test. The electrodes were placed with an electrode paste after cleaning the site with a spirit swab. The scalp electrodes were placed relative to bony landmarks. The active electrode was placed in the middle of the variation zone of the calcarine fissure at Oz, which is the highest point on the occiput. The reference electrode was placed at Fz or 12cm above the inion. The ground electrode was placed over the forehead Cz.VEP consists of series of waveforms of opposite polarity, a negative waveform (N) and a positive waveform (P), which is followed by the approximate latency. The latencies of the wave P100 (in milliseconds) and the peak to peak amplitudes of N70-P100 and P100-N155 (in microvolts) were considered from the recording.
Statistical Analysis Categorical data was represented in the form of frequency. Association between variables were assessed with Chi Square Test, Quantitative data was represented as mean and standard deviation. Comparison of variables has been done with Unpaired t test. ANOVA was applied to comparison of more than two groups. P value of <0.05 was considered statistically significant. Data was analyzed with IBM SPSS Version 22 for windows.
RESULTS
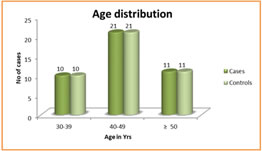
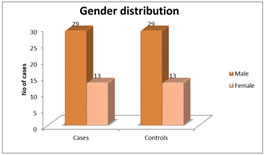
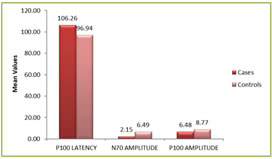
Figure 1 Figure 2 Figure 3 Figure 4 Figure 1: Shows the distribution of Age in among the study subjects; Figure 2: Shows the Gender Distribution in among the study groups; Figure 3: Shows the distribution of data of among three parameters in between cases and controls; Figure 4: Diagrammatic representation of placement of electrodes for VEP. Figure 1: Shows the 30 primary open glaucoma cases and 30 normal subjects were analysed for the study. Results were expressed in Mean ± Standard deviation. The age of the subjects ranged between 25 to 55 years. The significantly majority of cases were in 40-49 age group observed in among the study subjects. Figure 2: Shows the gender distribution in between the cases and healthy controls. The significantly more number of males were effected when compared to women among the study subjects. Figure 3 Shows the distribution of data of among three parameters in between cases and controls. There was a statistically significant increase in P100 latency in cases compared to the controls and also there was a statistically significant decrease in N70 and P100 amplitude in cases compared to the controls.
Table 1: Shows The data distribution among the study subjects by using Unpaired T – Test
DISCUSSION The present study was an attempt to compare visual evoked potentials (VEPs) in primary open angle glaucoma (POAG) patients and controls to find any differences in VEP latencies and amplitudes. VEP latencies are a measure of early glaucomatous damage before retinal ganglion cell death. In study by Rodarte et al. there was an increase in multifocal VEP latencies in open angle glaucoma as compared to the control.6 Mukesh Kumar Jha13 showed an increase in the pattern reversal latency N75 and P100 of cases was longer than the control but was not significant. All the pattern reversal VEP amplitudes (N75, N100, and N145) were significantly lesser in cases, similar to the studies done by Grippo et al.14 Bergua et al. concludedpeak time of the onset response was significantly (p<0.01) delayed in glaucomas when compared with normals (normals: 125.8±13 ms, glaucomas: 148.2±25.6 ms at 40 arc min).15 In a study by Thienpra siddhi et al. abnormal mfVEPs were detected in 20% of the eyes of glaucoma suspect patients and 16% of the eyes of ocular hypertensive patients. Significantly more mfVEP abnormalities were detected in glaucoma suspect patients than in normal controls.16 These of measurement, combined with its total objectivity, makes VEP a potentially useful screening technique, as well as an index of the progression of the disease17. Since our study was a cross sectional study, a follow-up study will give more information about the progression.
CONCLUSION There is a absolute increase in the latency of P100 along with significant decrease in the amplitude of P100 and N70 signifying the optic nerve damage secondary to primary open angle glaucoma.
REFERENCES
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||
 Home
Home