|
Table of Content - Volume 20 Issue 1 - October 2021
Incidence of retinopathy of prematurity among premature babies born at a tertiary care hospital - a longitudinal study with a short follow up
Alok B S1, Niveditha Alok Swamy2*, Mahesh I Magdum3
1Consultant Ophthalmologist and Cornea Surgeon, Department of Ophthalmology, Bangalore Nethralaya, Bengalore, Karnataka, INDIA. 2Assistant Professor, Department of General Medicine, MS Ramaiah Medical College, Bengalore, Karnataka, INDIA. 3Professor, Department of Ophthalmology, Jawaharlal Nehru Medical College, Belgaum, Karnataka, INDIA. Email: nitukerodi@gmail.com
Abstract Background: Retinopathy of prematurity (ROP) is a vasoproliferative disorder affecting premature infants. It is one of the most common causes of visual loss in children and can lead to lifelong vision impairment and blindness. Materials and Methods: All preterm infants born with a birth weight of <1500 grams and/or <= 32 weeks of gestation at KLE’S Dr.Prabhakar Kore Hospital and Medical Research Centre, Belgaum. Informed/written consent of parents was taken after explaining in detail about the methods and procedures involved in the study in their own vernacular language. Ethical clearance was obtained. Results: 100 babies who fulfilled the inclusion criteria were screened and 16 babies were found to have ROP. The incidence of ROP in this study was found to be 16%. Out of 16 babies with ROP, only 3 babies (18.75%) were in stage 1, 11 babies (68.75%) were in stage 2,1 baby (6.25%) was in stage3 and 1 baby (6.25%) developed APROP. Out of 100 babies screened, 51 were male and 49 were female. Among 51 male babies 8 (15.69%) developed ROP and out of 49 female babies 8 (16.33%) had ROP. In this study gender did not significantly influence the incidence (p=0.928) of ROP. Conclusion: The present study reflects the problem of ROP in a tertiary care centre. The incidence of ROP in the present study is 16%. Out of 16 babies with ROP, only 3 babies (18.75%) were in stage 1, 11 babies (68.75%) were in stage 2,1 baby (6.25%) was in stage3 and 1 baby (6.25%) developed APROP. In our opinion, the effective management of retinopathy of prematurity requires a team effort of the neonatologist, ophthalmologist and the NICU staff. Along with regular screening, an effective control of oxygen delivery, reduction of apneic spells and their early recognition and effective management of RDS are required. Key Words: Retinopathy of prematurity, APROP, RDS, apneic spells
INTRODUCTION Retinopathy of prematurity (ROP) is a vasoproliferative disorder affecting premature infants. It is one of the most common causes of visual loss in children and can lead to lifelong vision impairment and blindness.1 There are approximately 45 million blind people in the world today out of which, 30% are in Asia. Of the total blindness, childhood blindness accounts for 4%. It is estimated that there are about 1.4 million blind children, 1 million of whom live in Asia. India shares 20% of the world’s childhood blindness1. ROP afflicts over 3,00,000 infant’s worldwide.2 In developing countries like India the incidence of ROP has been reported at 24 – 47 % among high risk preterm infants.3 It is important not only in terms of economic burden but its severe social implication, which is very long in terms of blind years.2 In the context of our country we are sitting on the summit of two volcanoes- one where all latest state of art health care is available and the other where even minimal basic health care is unavailable. ROP is known to grow in both these conditions. Among the preventable causes of blindness in children (57%), ROP figures very high in the agenda. Low birth weight and gestational age were found to be the most important risk factor for the development of ROP.3 With neonatalogical units equipped with the state of art technological background and highly qualified personnel providing optimum care of extremely immature newborns, ROP incidence is on a rise. By early detection and timely intervention, blindness due to ROP is preventable.4,5 The purpose of this study is to know the incidence of ROP and to correlate it with maternal and neonatal risk factors.
OBJECTIVES OF STUDY Primary objective:To know the incidence of Retinopathy of Prematurity in preterm infants with birth weight ≤ 1500 grams or gestational age ≤32 weeks. Secondary objective: Relationship between development of ROP and the risk factors.
MATERIALS AND METHODS Source of Data: All preterm infants born with a birth weight of <1500 grams and/or <= 32 weeks of gestation at KLE’S Dr.Prabhakar Kore Hospital and Medical Research Centre, Belgaum. Method of collection of Data: A data collection instrument was used in which data was collected: 1. By interview of parents. 2. Hospital records. 3. Examination of the infant. Study Design: Longitudinal study with a short follow-up Study period: One year three months -1stJanuary 2011 to 31stMarch2012. Sample size: Sample size for Incidence with specified confidence level and specified relative precision
Z = specified confidence level (95% i.e.= 1.96) d = relative precision( 20% ) n = 97 ≈ 100 Sample size = 100 Inclusion criteria: Premature infants born ≤ 32 weeks and/or birth weight ≤1500 grams14. Babies b/n 1501-2000grams and/or 33-35weeks who are at higher risk of developing ROP106 like, Respiratory Distress syndrome, Sepsis, Multiple blood transfusions, Multiple birth’s, Apneic Episodes, Intraventricular haemorrhage. Exclusion criteria: Babies who did not consent for the study. Babies who died before full vascularisation of retina. Babies who did not complete the follow up protocol for other reasons were excluded Consent and ethical clearance: Informed/written consent of parents was taken after explaining in detail about the methods and procedures involved in the study in their own vernacular language. Ethical clearance was obtained. Definition of risk factors Apnoea: It is defined as a cessation of respiration for > 20 sec. which required resuscitation with bag and mask, with 100% O2. Sepsis: It was diagnosed by clinical picture, changes in leukocyte count, elevated CRP and positive blood culture. RDS: Presence of at least two of the following criteria: Respiratory rate > 60/min in a quiet and resting baby. Sub costal/ intercostals recessions. Expiratory grunt. Place of screening: All eligible babies were screened at Neonatal Intensive Care Unit. Preparation of the child: The pupils were dilated with a mixture of Phenylephrine 2.5% and Tropicamide 0.5% instilled 3 times at 10 mins interval about 1 hour before the scheduled examination. Resistance to dilation was noted. Care was taken to wipe off any eye drops with sterile cotton that come out of eyes to cheeks and not to feed the baby immediately before examination as the child might vomit or aspirate. Instruments used: Codless Indirect ophthalmoscope with 20D lens. Pediatric wire speculum. Scleral indentor. First examination: The following time table was used for screening.
Table 1: Timing of First Screening Eye Examination Based on Gestational Age at Birth107
First screening examination was carried out at 31 weeks of gestation or 4 weeks of age, whichever was later. For this purpose, gestational age was calculated from the last menstrual period. Follow up protocol: If no ROP was detected at initial examination, the infants were re-evaluated once every two weeks until vascularisation was complete. If ROP was detected, the examinations were performed weekly for stage 1-2 disease and more frequently for stage 3 disease, till the disease started resolving or progressed to threshold stage. Babies showing evidence of regression were followed up till vascularisation was complete. Babies progressing to threshold stage were referred. Procedure: All preterm babies who satisfied any one of the inclusion criteria were taken up for the study. The babies were enrolled into the study at birth. Parents were explained the nature of the examination and informed consent was taken. Demographic history and risk factors like respiratory distress syndrome, sepsis, multiple blood transfusions, multiple births, apneic episodes and oxygen given was documented using a data collection instrument. First examination was done at 4 weeks post natal age (age in weeks after birth) by taking all aseptic precautions in a temperature controlled room in the presence of a neonatologist.
Indirect ophthalmoscopic examination was done. One drop of topical paracaine eye drops was used to anaesthetise the cornea. A pediatric wire speculum was used to keep the eyelids apart. After decreasing the room illumination the anterior segment was first visualized to look for tunica vasculosa lentis, pupillary dilatation, lens and media clarity. Then the posterior pole was examined for any Plus disease. A scleral indenter was used to visualize the periphery. The periphery was examined in all clock hours to look for the extent of changes from nasal to temporal retina. Care was taken not to put too much pressure on the globe. During examination, untoward neonatal complications were looked for and managed appropriately. The changes in the retina were graded according to the International Committee for the Classification of Retinopathy of Prematurity (ICROP) guidelines-200515. Method of Statistical Analysis: The following methods of statistical analysis have been used in this study.
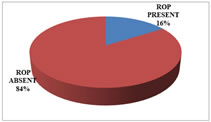
RESULTS INCIDENCE100 babies who fulfilled the inclusion criteria were screened and 16 babies were found to have ROP. The incidence of ROP in this study was found to be 16%. Table 1: Incidence of ROP
Figure 1: Incidence
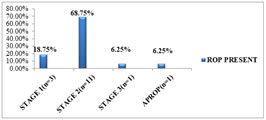
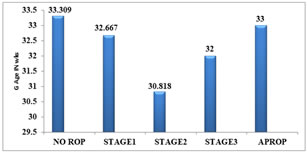
STAGES OF ROP Out of 16 babies with ROP, only 3 babies (18.75%) were in stage 1, 11 babies (68.75%) were in stage 2,1 baby(6.25%) was in stage3 and 1 baby(6.25%) developed APROP.
Table 2: RETINOPATHY OF PREMATURITY
Figure 2: STAGE OF ROP SEEN IN THE STUDY GROUP
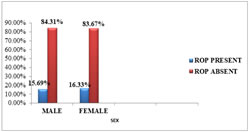
SEX DISTRIBUTIONOut of 100 babies screened, 51 were male and 49 were female. Among 51 male babies 8 (15.69%) developed ROP and out of 49 female babies 8 (16.33%) had ROP. In this study gender did not significantly influence the incidence (p=0.928) of ROP. Table 3: SEX VS ROP
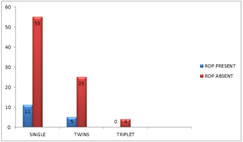
Figure 3: SEX DISTRIBUTION IN THE PRESENT STUDY BIRTH ORDEROut of 100 babies, 66 babies were singletons, 30 babies were twins and 4 babies were triplets. Out of 66 singletons, 11 babies developed ROP. Only 5 of the 30 twins developed ROP and no triplet developed ROP. Birth order was not found to be significantly associated (p=0.971) with ROP in the present study.
Table 4: BIRTH ORDER VS ROP
Figure 4: BIRTH ORDER AND ROP
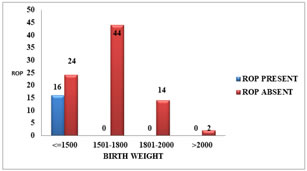
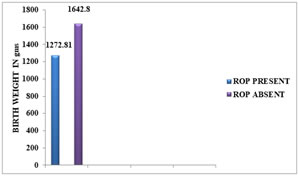
BIRTH WEIGHT AND ROP: The birth weight of the ROP babies ranged from 1000-1500 gm (mean 1272.81 ±143.67 gm), while that of non-ROP babies ranged from 1100-2100 gm (mean 1642.80 ± 216.60 gm). Lower birth weight was significantly associated with increased incidence (p = <0.001) of ROP. The incidence of ROP was 40% in babies weighing ≤ 1500gm at birth.
Table 5: DISTRIBUTION AS PER BIRTH WEIGHT
Table 6: BIRTH WEIGHT AND ROP
Figure 5: DISTRIBUTION OF ROP AS PER BIRTH WEIGHT Figure 6: DISTRIBUTION OF MEAN BIRTH WEIGHT TO ROP
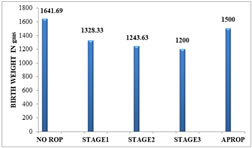
Table 7: BIRTH WEIGHT AND STAGE OF ROP
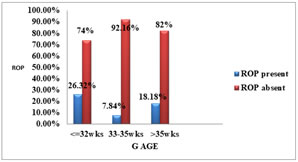
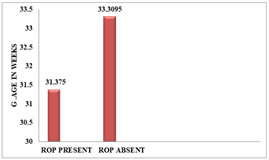
Figure 7: Distribution Of Mean Bt Wt To Stages of ROPGESTATIONAL AGE AND ROP:The gestational age of the ROP babies ranged from 28 -34 weeks (mean 31.38 ±1.63 weeks), while that of non-ROP babies ranged from 30-38 weeks (mean 33.31 ± 1.74 weeks). The incidence of ROP was 26.32% in babies born ≤ 32 weeks of gestational age.
Table 8: DISTRIBUTION OF GESTATIONAL AGE
Table 9: GESTATIONAL AGE AND ROP
Figure 8: FREQUENCY OF ROP TO GESTATIONAL AGE Figure 9: DISTRIBUTION OF MEAN GESTATIONAL AGE TO ROP
Table 10 :
Figure 10: DISTRIBUTION OF MEAN GESTATIONAL AGE TO STAGES OF ROP
Table 11: FRESH FROZEN PLASMA VS ROP
Table 12: PREGNANCY INDUCED HYPERTENSION VS ROP
Table 13: FETAL DISTRESS VS ROP
Table 14: HYPOTENSION VS ROP
Table 15: ANAEMIA VS ROP
Table 16: METABOLIC ACIDOSIS VS ROP
DISCUSSION Retinopathy of prematurity (ROP) is a vasoproliferative disorder affecting premature infants. It is one of the most common causes of visual loss in children and can lead to lifelong vision impairment and blindness.6 Out of 45 million blind people in the world today, there are about 1.4 million blind children. ROP afflicts over 3,00,000 infant’s worldwide2. In developing countries like India, the incidence of ROP has been reported at 24-47 % among high risk preterm infants. It is important not only in terms of economic burden, but in its severe social implication, which is very long in terms of blind years. Among the preventable causes of blindness in children (57%), ROP figures very high in the agenda. Low birth weight and gestational age were found to be the most important risk factors for the development of ROP.7 We screened babies admitted to our NICU with birth weight ≤ 1500g and gestation ≤ 32 weeks. Infants with birth weight >1500g and gestation more than 32 weeks were screened only if they had additional risk factors. In an article, Chawla, et al. has suggested the same screening criteria. INCIDENCE: With neonatalogical units equipped with the state of art technological background and highly qualified personnel providing optimum care of extremely immature newborns, ROP incidence is on a rise. The overall incidence in the present study was found to be 16% with only one case of severe ROP (APROP). It is of current knowledge that aggressive posterior ROP seems to occur especially among smaller and more immature patients. In our study the baby which developed APROP, the birth weight was 1500 gms and gestational age was 33 weeks. In the CRYO-ROP study the incidence of the disease in a group of premature newborns with a birth weight <1251gms was 65.8% and 81.6% for infants of less than 1000 g birth weight16. In the ETROP (multi centric) study done 15 years later the overall incidence of ROP was found to be 68% in babies with birth weight <1251 gms. The overall incidence of more-severe ROP (prethreshold) was 36.9% among infants with ROP in the ETROP Study, whereas the incidence was 27.1% for patients in the CRYO-ROP Study who developed ROP.8 The incidence of severe form of the disease (Threshold disease) is decreasing. Agarwal and co-workers found a drop from 46 to 21% in their study over a period of 7 years. A Danish study found a statistically significant decrease in incidence of ROP in infants weighing more than 1250gm. Similar observations were made in multicentre study in UK. Nair and colleagues, Gupta and co-workers found no cases of ROP in babies weighing more than 1250gm. Post Conceptional Age at First Examination: Post Conceptional Age at First Examination among ROP babies ranged from 33 – 38 weeks (mean 35.69 ± 1.58 weeks), while that of non-ROP babies ranged from 34 – 41 weeks (mean 37.38 ± 1.78 weeks). Early examination was significantly associated with chances of early detection of ROP (p = 0.016) in the present study. RISK FACTORS: In our study Birth weight, Gestational age, Oxygen, Respiratory distress Syndrome, Transfusion and Apnoea of Prematurity were found to be significant risk factors on Chi Square analysis. On Univariate analysis, risk factors associated with ROP were Oxygen, RDS andTransfusion(FFP/Platelet) whereas on multivariate analysis, RDS was found to be an important risk factor. Using the forward method Oxygen and Transfusion were also found to be significant on Multivariate analysis. Birth Weight and Gestational age: In our study both low birth weight (p=<0.001) and prematurity (p= 0.004) were found to be significant risk factors for the development of ROP. The birth weight of the ROP babies ranged from 1000-1500 gm (mean 1272.81 ± 143.67 gm), while that of non-ROP babies ranged from 1100-2100 gm (mean 1642.80 ± 216.60 gm). Lower birth weight was significantly associated with increased incidence (p = <0.001) of ROP. The incidence of ROP was 40% in babies weighing ≤ 1500gm at birth. The gestational age of the ROP babies ranged from 28 -34 weeks (mean 31.38 ±1.63 weeks), while that of non-ROP babies ranged from 30-38 weeks (mean 33.31 ± 1.74 weeks). The incidence of ROP was 26.32% in babies born ≤ 32 weeks of gestational age. Gestational age was found to be a significant risk factor for the development of ROP (p=0.004) in this study. Oxygen: In our study oxygen was found to be significant risk factor for the development of ROP on Chi square analysis (p=0.005), on univariate analysis and also on multivariate analysis. Out of 100 babies screened 59 were given O2 and 15 (25.42%) babies developed ROP. The causal link between ROP and supplemental oxygen has been confirmed by controlled trials and clinical studies. Gunn analyzed data from their low birth weight survivors and found a significant association between the more severe grade of cicatrical disease and duration of oxygen therapy. Kinsey noted that concentration of oxygen administered was significantly associated with ROP in infants under 1200gm. When comparing mean Pa02 levels in normal infants and in ROP infants, he found differences only in babies of low birth weight and only Pa02 levels greater than 150mmHg. However, a safe level of oxygen usage has not been defined, keeping Pao2 <100 mm Hg is recommended preferably between 50 and 70mm Hg and saturation between 90-95%. Preliminary work has suggested that continuous oxygen monitoring may reduce the incidence of ROP. RESPIRATORY DISTRESS SYNDROME AND ROP: Out of 100 babies screened 55 had RDS and 14 (25.45%) babies developed ROP. RDS was found to be a significant risk factor for the development of ROP (p = 0.009). RDS was also found to be significant on univariate and multivariate analysis. Gupta et.al reported ROP in 33% of babies with RDS. Gupta et.al, Akkoyun et.al also found RDS to be a significant risk factor for the development of ROP. TRANSFUSION: Out of 100 babies screened, 27 were given transfusion (either FFP / Packed cell/ Platelet) and 9(33.33%) developed ROP. Transfusion was found to be a significant risk factor for the development of ROP in this study (p=0.004). Transfusion was also found to be significant on univariate and multivariate analysis in our study. A potential role for blood transfusions and /or anaemia in the pathogenesis of retinopathy of prematurity (ROP) has been suggested. However a number of prospective, randomised, trials have failed to show a link between ROP and anaemia or transfusions. Dutta et.al reported that packed cell and double volume exchange transfusion in the neonatal period as a major risk factor for the development of ROP.9 Apnoea of prematurity: Out of 100 babies screened, 24 babies had AOP and 7(29.17%) developed ROP. AOP was found to be a significant factor for the development of ROP in this study (p=0.044). Agarwal et. al (54.1%), Gupta et.al(54.5%) found apnea to be a significant risk factor for the development of ROP. In a study by Kim et al., they found that apnoea independently increased the incidence of ROP. Furthermore, frequent apnoeic attacks increased the progression of pre-threshold ROP to threshold ROP. A higher incidence of hypoxemia and apnoeic episodes requiring bagging was found among infants with severe ROP than in a control group. Similarly in a study by Chen et al. they found that apnoea was one of the independent risk factors of ROP. Its appropriate management might reduce the incidence of ROP.10 Other risk factors: In our study we did not find sepsis, phototherapy, PIH, fetal distress, hypotension, anaemia and metabolic acidosis to be associated with ROP as found in other studies. LIMITATIONS OF THE STUDY: The sample size is small and may not represent all premature babies. To know the true incidence and risk factors involved it is advisable to undertake larger study over a period of years.
CONCLUSION The present study reflects the problem of ROP in a tertiary care centre. The incidence of ROP in the present study is 16%. Out of 16 babies with ROP, only 3 babies (18.75%) were in stage 1, 11 babies (68.75%) were in stage 2,1 baby (6.25%) was in stage3 and 1 baby (6.25%) developed APROP. In our opinion, the effective management of retinopathy of prematurity requires a team effort of the neonatologist, ophthalmologist and the NICU staff. Along with regular screening, an effective control of oxygen delivery, reduction of apneic spells and their early recognition and effective management of RDS are required.
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home