Official Journals By StatPerson Publication
|
Table of Content - Volume 5 Issue 3 - March 2018
Bacterial flora and their antibiotic susceptibility patterns in open fracture wounds at a tertiary hospital of north India
Shiraz M Bhatty1, Kapil Bansal2*, Anshul Dahuja3, Rajesh Paul4
1Associate Professor, Department of Orthopaedics, Adesh Institute of Medical Sciences and Research, Bathinda, Punjab, INDIA. 2Associate Professor, 3Assistant Professor, Department of Orthopaedics, GGS Medical College and Hospital, Faridkot, Punjab, INDIA. 4Professor, Department of Orthopaedics, Christian Medical College and Hospital, Ludhiana-141008, Punjab, INDIA. Email: kapilortho@gmail.com
Abstract The changes in pathogenic microbiological flora and the emergence of bacterial resistance have created major problems in the management of open fractures. A better understanding is required of the patterns or predilection of organisms and thus anticipating infection by a particular organism. Hundred and seven open fractures wounds of long bones were studied prospectively over a period of one year. Wound swabs were obtained and sent for cultures at regular intervals i.e.; at Pre-debridement, intra-operative, post debridement, at first dressing and then every week. The infecting organism and its antibiotic susceptibility were noted. Most of the infections were caused by Gram-negative organisms 64.7%, commonest being Pseudomonasspp (36%) which was resistant to most antibiotics and showed maximum sensitivity to piperacillin (85%).A shift in the bacterial flora was noted after the 2nd week from Gram-negative to predominantly Gram-positive organisms. Among the Gram-positive organisms, 93.5% were Staphylococcus aureus, 58.6% of which were methicillin resistant. Use of a broad spectrum antibiotic during the initial phase of management could prevent a change in the bacterial flora in later stages and thus decreasing chances of bacterial resistance. However the final selection of antibiotic should be tailored according to the type of fracture, level of contamination, soft tissue status, and most importantly the prevailing infection and culture sensitivity patterns in the hospital. Key Words: Bacterial flora, Open fractures, Antibiotic
INTRODUCTION Open or compound fractures are fractures that communicate with the outside environment through a skin wound. According to Gustilo-Anderson classification open fractures are classified in to three major types (type III has three subtypes) based on mechanism of injury, the degree of soft tissue damage, the configuration of the fracture, and the level of contamination. Treatment for open fractures is a challenge. Prevention of infection is one of the prime objectives in management of open fractures1. Sepsis occurring in between 2% and 25% of all open fractures, leads to significant morbidity. Seventy percent of open fracture wounds are believed to be contaminated at the time of injury. Deep fracture-site infections can lead to chronic osteomyelitis, non – union, loss of function, or even limb loss2. The contaminating bacteria originate from both skin and environment. In some cases the organism is not present at the time of injury, and the wound becomes inoculated later. Based on the types of organisms causing infection compared with those seen on early wound cultures, several authors have proposed that many infections of open fracture wounds are nosocomial. Wound infecting pathogens differ from country to country3,4. The source of an infecting organism may be one of the following: a) endogenous, from patient’s own flora; b) exogenous, from another patient or a member of the hospital staff or from the inanimate environment of the hospital; c) environment (air, food, water, soiled linen, hospital waste etc) d) Contamination of wounds at the time of injury5. This study was designed to determine the microbiology of the open fracture wounds at the time of initial treatment and development of subsequent infections. An attempt has also been made to study the bacterial flora in relation to various factors that affect the biology and outcome of open fractures which could help us in anticipating infection by a particular organism and thus providing appropriate antibiotic therapy.
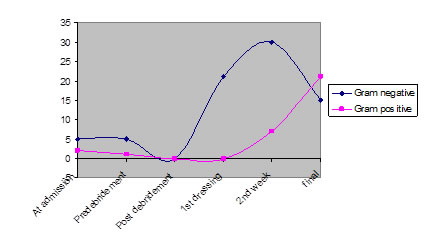
Materials and methods This prospective study was done over a period of one year in the Department of Orthopaedics at a tertiary care hospital in north India. Patients brought to our trauma centre with open fractures of long bones were included and were classified according to the Gustilo and Andersonclassification (6). Patients with Gustilo’s grade IIIC fractures requiring an amputation were excluded from the study. A wound swab was obtained at the time of presentation, tetanus prophylaxis, and irrigation of the wound was done. A third generation cephalosporin was usually started in Gustilo’s grade I and II fractures and an amino glycoside was usually added in grade III open fractures. Primary wound debridement was done in the operation theatre under appropriate anaesthesia with appropriate stabilization of the bone. Wound cultures were obtained prior to and after debridement. Regular wound care was done and wound cultures were sent after 24hours of debridement at the time of first dressing and then subsequently every week. Any change in the intra-venous antibiotics was recorded. Microbiological Methods: For all wound cultures two swab sticks were obtained in a culture tube. One swab was used to obtain smear by Gram staining. The second swab was used for inoculation on Blood agar and Mac Conkey agar and the plates were incubated at 37 degrees Celsius overnight. The swabs were assayed for the predominant organisms found in culture and the microbial sensitivity/resistance patterns according to standard techniques7. Antibiotic susceptibility testing was done by Disc diffusion method and measuring diameter of zone of inhibition as described by Kirby Bauer method on Mueller Hinton Agar (MHA). RESULTS Out of hundred and seven open fractures wounds, pathogenic bacteria were found in 43.9% cases. Most of the patients (27.1%) were in the age group of 20-30 years with a mean age of 33.93. The male female ratio was 9:1. Road traffic accident accounted for highest percentage of injuries (77.6%). Most of patients (46.6%) in our study had grade IIIB open fractures. The bacterial profile at different stages of wound care is shown in Table 1. The bacterial profile in relation to various factors like grade and site of fracture and duration of presentation after injury are sited in Tables 2 and 3. The antibiotic susceptibility of various pathogenic bacteria is detailed in table 4. Most of the initial wound cultures showed growth of Gram-negative organisms (71.4%) as compared to their only 40% growth in the final cultures. On the other hand Gram-positive organisms were present in only 28.6% of patients at admission as compared to their 60% growth in the final culture. This change occurred after the 2nd week as evident from the graph (Fig.1).
Table 1: Bacterial profile at different stages of wound care
Table 2: Bacterial flora in different grades of open fractures
Table 3: Bacterial flora in relation to duration and site of injury
Table 4: Antibiotic susceptibility patterns (%)
Figure 1: Graph showing bacterial flora in different stages of wound management
Discussion Infection rates among open fractures, ranging from 2%-25% have reported in the past8. In this study we observed a high infection rate i.e. 43.9%. Some of recent studies by Ikem et al9 and Sen et al10 have similarly reported high incidence of infection i.e.; 45.8% and 45% respectively. The high infection rate in our study could be explained by the predominance of Grade III injuries and high velocity trauma which are likely to have a higher level of tissue contamination. Microbiology of open fractures has been changing since the late 70s. Coagulase-positive Staphylococcus aureus was predominant in the 70s and early 80s11,12. However, over past several decades the pattern of infection has been changing and gram negative bacteria are becoming more and more common5. Our study shows that gram negative infections continue to be a major threat and were isolated from 64.7% cases. Pseudomonas spp (23.3%) was the commonest gram negative bacteria isolated in our study. Gram-positive organisms accounted for 26.7% of infections and the predominant Gram positive organism was Staphylococcus aureus (93.5%), 58.6% of which were methicillin resistant. Recent studies by Akinyoola et al13 and Ako-nai et al14 similarly reported predominance of gram negative bacteria i.e.; 40.5% and 53.2% respectively. Akinyoola et al13 also observed pseudomonas spp (11.2%) to be the predominant gram-negative organism. However Ako-nai et al14 found E-coli (12.8%) to be the commonest gram-negative organism. The use has become almost universal and widespread in management of open fractures. Recently it was hypothesized that this strategy may lead to selection of more virulent and also antibiotic resistant bacteria which subsequently would result in an increased infection rate over the years15. Our study clearly shows that Pseudomonas spp is resistant to most antibiotics as also observed by Agarwal et al9. Various studies have been conducted till date, studying the bacterial flora in open fracture wounds but there is a dirth of studies which evaluate the flora in relation to various factors that affect the biology and outcome of open fractures. In our study, we had made an attempt to study the bacterial profile in relation to some of these factors which could probably help in selection of prophylactic antibiotics. We observed that Acinetobacter spp (23.3%) and Pseudomonas spp (54.5%) were more likely to be isolated from open fractures of the lower and upper limbs respectively. Staphylococcus aureus was predominant isolate among grade I (100%), IIIA (37.5%) and IIIB (22%) groups whereas Grade II (50%) and IIIC (33.3%) fracture wounds had maximum infection with Pseudomonas spp.. Fresh wounds reaching within 6hrs were more likely to grow Acinetobacter spp and Pseudomonas spp whereas unusual pathogens like E-coli and Proteus were isolated in increasing frequency with increasing duration of injury which could probably be the flora prevalent in the hospital were they had received initial treatment. A shift in the bacterial flora was also noted from Gram-negative to Gram-positive after the second week. This change can be attributed to the use of antibiotics during the initial phases of wound management which are more effective against Gram-negative organisms. Thus suppressing the Gram-negative organisms and but leaving behind the Gram-positive organisms to flourish later in the course of wound management. Also, debridement and irrigation change the ecology of local wound and finally another possibility is that the infecting bacteria are nosocomial. It could thus be concluded from our study that most of bacterial infections in open fracture wounds are acquired during the course of treatment and the isolated bacteria would depend upon the microbiologic environment of the institution; identifying the patterns or predilection of organisms and anticipating infection by a particular organism in that institution might be worthwhile. Secondly a change in bacterial flora from Gram-negative to Gram-positive occurs usually in the second week. Broad spectrum antibiotic during the initial phase of wound management might prevent this change and early coverage of the wounds within the first week would further decrease the incidence of nosocomial infections. However, the final selection of antibiotic treatment should be determined by the previous experience of organisms isolated and sensitivity studies done from open fracture wounds in each institution. Setting up of infection control programmes in each institution could prove worthwhile especially in developing countries where they are still non-existent or in their infancy.
REFRENCES
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home