Official Journals By StatPerson Publication
|
Table of Content - Volume 7 Issue 3 - September 2018
A comparison of graft union time in vascularized and avascular fibula for reconstruction in tumors of the appendicular skeleton
Joji Joshua Philipose1, Renjit Kumar Jayachandran2*
1Assistant Professor, Department of Orthopaedics, Believers Church Medical College Hospital, St.Thomas Nagar, Kuttappuzha, Thiruvalla, Kerala, INDIA. 2Sr. Consultant, Division of Spine & Musculoskeletal Oncology, Department of Orthopaedics, Khoula Hospital, Muscat, OMAN. Email: joji@bcmch.org, drrenjit@yahoo.com
Abstract Background: Resection of the tumor by a limb-sparing procedure followed by restoration of soft tissues makes a successful limb salvage surgery. Fibulais usually used because of its advantages. The reconstruction with fibula graft can vascular or avascular. The objective of the study is to assess and compare the graft union time in vascularized and avascular fibula when used for reconstruction in patients treated with wide resection of tumors of the appendicular skeleton. Methods: The study has been conducted in 17 patients who underwent resection of appendicular musculoskeletal tumors with fibular reconstruction. Retrospective and prospective comparative study was done. All clinical and surgical details were recorded. The patients were followed up regularly and a comparison was done between vascularized and avascular fibula regarding graft union time. Mann-Whitney Test was used to assess the difference in the union time between vascular and avascular fibula graft. Fisher’s Exact Test was done to assess significant differences in the presence or absence of fibular hypertrophy between vascularized and avascular fibula graft. Results: The patients included six cases with giant cell tumor, five patients with osteosarcoma, two with Ewing’s sarcoma and 1 each having fibrous dysplasia, osteoid osteoma, chondrosarcoma and metastasis from thyroid carcinoma. The chondrosarcoma and one case of giant cell tumor were cases of recurrence. Six patients had tumor in humerus, two in radius, seven people had in femur and one each in tibia and metatarsal (Age 7-58 Yrs). The mean resection length was 11.76 cm (6-19 cm). Among the 17 patients, 9 had vascularized fibula graft and rest of them had avascular fibula. The mean fibula length was 13.94 cm (7-24 cm). Fixation was done with appropriate implants and/or grafts. Fibula graft had united and hypertrophied in 7 patients out of which 5 of them were vascularized and 2 avascular ones. Bony union with no hypertrophy was seen in three vascularized and three of the avascular fibula grafts. 13 of 17 patients had solid union, two of them (avascular) united only at the distal graft site, one of them (vascular fibula) united only at the proximal site and one patient who had 75% resorption of fibula graft showed improvement in his functional score when compared to the preoperative status. Two patients succumbed after graft union has achieved. The mean union time of vascularized fibula graft was 6.63 months (3-12 Months). The mean union time of avascular fibula graft was 8 months (3-12 months). There was no statistical significance in the union time (Mann-Whitney test, p-value 0.415) and in the presence of hypertrophy (Fisher’s exact test, p value 0.413) when vascularized and avascular fibula groups were compared. Conclusions: Fibula grafting after resection of tumors of the appendicular skeleton offers a satisfactory technique in reconstructing circumferential bony defects. It provides good bony union and functional outcome. Even though the postoperative functional outcome is significant, there is no significant difference between vascularized and avascular fibula in terms of bony union and graft hypertrophy. Key Words: Fibula graft union, vascularized, Avascular, Reconstruction, Appendicular Skelton tumors
INTRODUCTION Resection of the tumor by a limb-sparing procedure followed by restoration of soft tissues makes a successful limb salvage surgery.1 Better diagnostic, staging, newer chemotherapy techniques have made limb salvage a viable option in a good number of patients. There are various reconstructive options that focus on maintaining limb length, function as well as cosmesis. These include endoprostheses, either alone or with allografts, osteoarticular allografts, allografts in arthrodeses, skeletal lengthening techniques, intercalary allografts and autografts using conventional avascular or free vascularized techniques. The decision over which surgical intervention to use depends on tumor-related factors, such as location, sizeand soft tissue conditionsas well as patient-related factors, such as age, personal preferences, and cosmetic requirements.1Though there are different sites for donor grafts, the fibula is usually used because of its good blood supply, straight, strong, cortical structure, available length and low donor site morbidity. Upto 30 cm defects can be grafted using fibula.2 After resection of the tumor defects, reconstruction can be done with fibula graft using either vascular or avascular graft.3 Vascularized grafting is particularly indicated in younger patients, diaphyseal defects, areas of poor soft tissue coverage, and after failed endoprosthetic or allograft reconstructions. An advantage of a vascularized fibular transfer is its ability to hypertrophy. Avascular fibular transfer is a simpler, less expensive and a shorter procedure than the use of vascularized grafts and allows remodelling of the fibula at the donor site. However, all limb salvage procedures carry the potential risk of infection, aseptic loosening, nonunion or delayed graft union and implant failureas well as donor site morbidity.4 Free vascularized fibular grafting (FVFG) was first reported in 1975 by Taylor et al. for reconstruction of large tibial defects and for use in the upper limb by Pho and Weiland et al. in 1979. Over the last decades, free fibular vascularized grafting has successfully been used for a wide range of conditions, including defects after tumor resection. Peroneal artery is used as the vascular pedicle in case of vascularized fibula graft.5 The use of non-vascularized fibula graft has been described in skeletal defects. The danger of resorption and the lack of biological activity have been thought to be a disadvantage of non-vascularized grafts.6 Various surgical site complications have been recorded with this procedure like wound infections, graft fracture nonunion, hardware issues, resurgeries and donor site morbidities like peroneal nerve palsy.7 The objective of the study is to assess and compare the graft union time in vascularized and avascular fibula in patients treated with wide resection of tumors of the appendicular skeleton.
MATERIAL AND METHODS The study has been conducted in Amrita Institute of Medical Sciences, Kochi, Kerala on all patients who have undergone resection of appendicular musculoskeletal tumors with fibular reconstruction. Retrospective and prospective comparative study was done. Data was collected using direct interview and perusal of electronic medical records, case sheets, x-rays, operative details and outpatient follow up notes. All clinical and surgical details were recorded. The patients were followed up regularly and a comparison was done between vascularized and avascular fibula. The patients were followed up for an average 30.3 months with a range between 9 and 65 months. Regarding graft union time the patients were followed up at 6 weeks, 3 months, 6 months and 12 months. Radiographs were available for all patients and were reviewed by the surgeon. Radiographs were assessed for evidence of union, hypertrophy, time of union, osseous resorption, implant failure and other complications. Bony union of the recipient site was defined as the presence of an indistinct or absent osteotomy line or external bridging callus or the presence of bridging bony trabeculae. Mann-Whitney Test was used to assess the difference in the union time between vascular and avascular fibula graft. Fisher’s Exact Test was done to assess significant differences in the presence or absence of fibular hypertrophy between vascularized and avascular fibula graft. The level of significance was set at p value 0.05. The SPSS software and Minitab software was used to analyze the data collected.
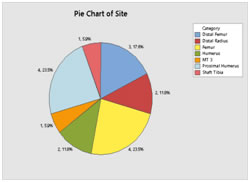
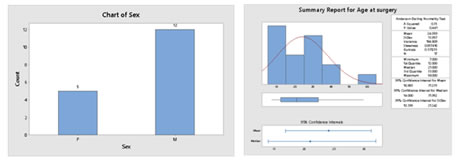
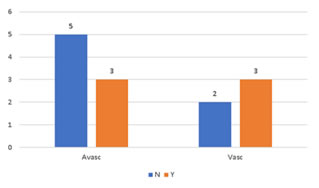
RESULTS The patients included six cases with giant cell tumor, five patients with osteosarcoma, two with Ewing’s sarcoma and 1 each having fibrous dysplasia, osteoid osteoma, chondrosarcoma and metastasis from thyroid carcinoma. The chondrosarcoma and one case of giant cell tumor were cases of recurrence. Six patients had tumor in humerus, two in radius, seven people had in femur and one each in tibia and metatarsal. (Figure 1) Figure 1:Site of surgery The mean age of the patients at the time of surgery was 24 with a range 7 to 58 years. Twelve of them were males and five were females. Six patients reported history of trauma prior to presentation. (Figure 2) Figure 2: Distribution of sex (a) and age (b) at surgery of the subjects
MRI scan was done in all patients except the one with fibrous dysplasia of metatarsal. Thirteen patients had reconstruction and four of them had arthrodesis.The mean resection length was 11.76 cm with a range of 6-19 cm. Among the seventeen patients, nine had vascularized fibula graft and rest of them had avascular fibula. (Figure 3) The mean fibula length was 13.94 cm with range of 7-24 cm. Figure 3: Type of Fibula 9 were Vascularised and 8 avascularized.
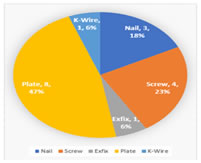
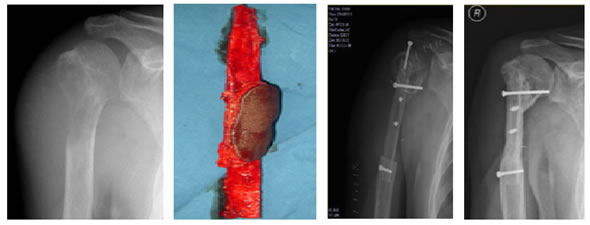
The fibula graft was held in place with plate and screws in eight patients, screws in four, nail in three, K-wire in one and external fixation in one patient. (Figure 4). Figure 4: Type of implants used Additional cancellous/cortico-cancellousbone grafting was done in the resected area after reconstruction in eleven patients. Among them, iliac crest autograft was used for eight patients and proximal tibia autograft in three patients. The autograft was augmented with allograft in five patients. In addition to the bone and soft tissue reconstruction, four patients had skin grafting over the surgical site. (Figures 5-8).
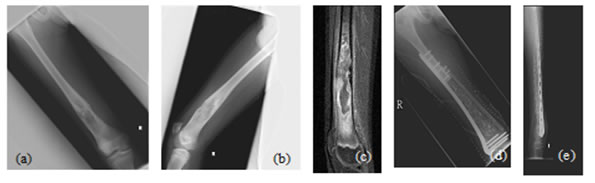
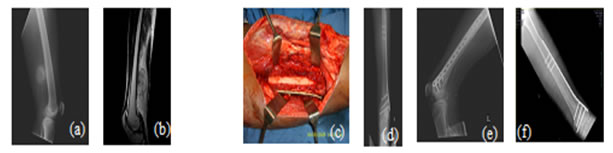
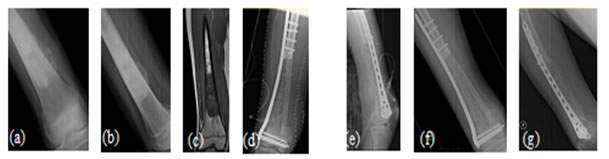
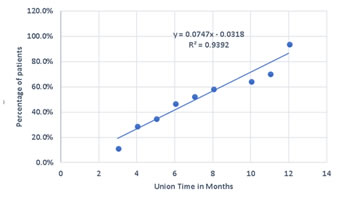
Figure 5: Case 1: (a) Pre-OP (b) Intra-OP (c) Immediate Post-OP (d) Post OP-65 Months Figure 6: Case 8: (a-c) Pre OP (d, e) Post-OP Figure 7: Case 13: (a, b) Pre OP (c) Intra OP (d, e) Immediate Post-OP (f) Post OP-2 years Figure 8: Case 16: (a-c) Pre-OP (d, e) Immediate post OP (f, g) Post OP-1 Year Intraoperative Issues: The resection in one patient was marginal. Yet there was no evidence of recurrence even when the patient was followed up after 30 months.The mean follow up of the patients was 30.3 months with a range of nine to sixty-five months. Nine patients had resurgery over the surgical site and one patient had a biopsy in a distant site of metastasis. One patient had local soft tissue recurrence near the primary site and two patients out of the study died due to additional lesions at a distant site.Two patients required radiotherapy. In one patient, radionuclide I-131 ablation was done was for the primary thyroid follicular carcinoma who had solitary metastasis in the distal femur and the other was for the metastatic (brain and spine) deposits that had osteosarcoma of proximal humerus. The latter patient later succumbed to the illness. Resurgery: Two cases where bony union was not achieved required bone grafting surgery. One was after 2 years 3 months which required implant revision also. The other was after 1 year 10 months. Chemotherapy was given for six patients and among them, two patients died of distant metastasis of which one patient had discontinued chemotherapy. Functional Outcome: Functional outcome was assessed and compared using Musculoskeletal Tumor Society Score (MSTS). There was a significant difference in the MSTS score between preoperative and postoperative function. The scores improved postoperatively in most of the patients. Graft Union:Analysis of graft union was done in all patients. Fibula graft had united and hypertrophied in seven patients out of which five of them were vascularized and two avascular ones. Bony union with no hypertrophy was seen in three vascularized and three of the avascular fibula grafts. In one case where avascular fibula was used, there was 75 % resorption of the graft.Among the total 17 patients, 13 patients had solid union, two of them (avascular) united only at the distal site, one of them (vascular fibula) united only at the proximal site and one patient had resorption of fibula graft. The patient who had 75% resorption of the graft had an improvement in his functional score when compared to the preoperative status. Among the 13 patients that had strong fibula graft union eight were vascularized and five were avascular fibula. Two patients succumbed after graft union has achieved. (Figure 9) Figure 9: Fibula Graft union in Vascular and Avascular (number of patients) The fibula graft union timewas assessed during follow up of 6 weeks, 3 months, 6 months and 12 months in most of the patients. Graft union was assessed by a single surgeon. One patient had resorption of fibula. In two patients who had proximal site non-union, the distal site united in six and eight months respectively. The above three patients had avascular fibula graft. In one patient who had distal site non-union, the proximal site was united in 12 months. Vascularized fibula graft was used in this patient. Rest of the 13 patients had a mean union time of 7.15 months with a range of 3 months to 12 months. The correlation of ‘time of union’ had shown a linear trend with ‘percentage of patients, with an R2 of 0.9392 (Figure 10)The mean union time of vascularized fibula graft was 6.63 months with range of 3 months to 12 months. The mean union time of avascular fibula graft was 8 months with a range of 3 months to 12 months. There was no statistical significance (Mann-Whitney test) in the union time when vascularized and avascular fibula groups were compared (P-value 0.415). There was no significant difference (Fisher’s exact test) in the presence of hypertrophy between vascularized and avascular fibula (P value 0.413). (Table 1-3)Six patients had undergone neoadjuvant as well as adjuvant chemotherapy. Among them, one patient had proximal site graft non-union. Excluding those patients who had graft site non-union, a comparison was done regarding time to union between patients who underwent chemotherapy and those who did not have chemotherapy. The mean time to union for those who had chemotherapy was 7 months. For those patients without chemotherapy, the mean time to union was 7.5 months. It seemed chemotherapy did not have any adverse effect on the graft union time in this study.
Figure 10: Scatter plot of union time in months vs the percentage of patients
Table 1: Union Time between Vascular and Avascular Fibula grafts
Refracture:One patient developed resorption of the graft and he fractured the fibula graft 5 months after surgery which was treated in sling. The same patient had foot drop as donor site morbidity also. Apart from him, only one more case had fracture of medial fibula strut and all condylar screws after 27 months of index surgery for which implant revision and bone grafting was done and the graft had shown union after 2 months of second surgery.
Table 3: Presence of Hypertrophy between vascularized and Avascular fibula (n (%)).
All values are Count (%). Fisher’s Exact Test (1- sided) value = 0.413 (Association is not statistically significant).
DISCUSSION Fibula reconstruction after resection of tumors of appendicular skeleton is well established. For larger defects, free vascularized bone graft has a great potential to maintain good functional ability in the affected limb, a factor which is particularly important in younger patients8. Reports have suggested that this technique also produces a more rapid skeletal union because of graft healing by secondary healing rather than ‘‘creeping substitution’’9. When compared to avascular techniques, it also displays increased initial graft strength, and an increased ability to respond physiologically to biomechanical loading. This has several clinical advantages, including a decreased rate of complications such as infection, fracture, and pseudoarthrosis, and a reduced immobilization time after implantation. Being a vascular graft, the adequate penetration of antibiotic concentration is also not hindered.Fibula grafting after resection most often have showed better functional results at later follow up. Union between transplant and recipient bone occurs from 6 to 12 months. Many of the studies showed an average bony union in 7.6 monthsin case of vascular fibula grafts. Ennekinget al10,11 found primary union in 63% of the long bone reconstructions within the first 12 months and Yadav, union after 8 to 10 months in 60%.We achieved a primary union in 76.47 % of the reconstructions in 12 months follow up. In our study, there was no significant difference in bony union time between vascularized and avascular fibula.Krieg and Hefti reported a median bony union time of 24 weeks in case of nonvascularized fibula grafts. Dell et al found no substantial difference in incidence of union between vascularized and nonvascular dog fibula graft. In an experimental animal study bridging segmental defects of the radius and ulna, nonvascularized grafts hypertrophied nearly as much as the vascularised fibulae10. The difference in the quantum of hypertrophy between vascular and avascular fibula was also not significant in our study.Compared to avascular fibula grafting, free vascular grafting is a complex procedure requiring a skilled microvascular surgeon, the infrastructure for carrying out the procedure and longer operative duration thereby increasing the cost of the procedure. On the other hand avascular fibula grafting can be carried out with a gentle learning curve and operating time.The main disadvantage associated with fibula grafting is the low post-operative loading capacity experienced by patients, which inevitably results in a relatively long period of immobilization as against endoprosthetic reconstruction.10 The free vascular fibula graft can bridge the defect and achieve bone union even in concurrent radiotherapy or chemotherapy. Malizoset al 16 reported primary union in 86% of oncologic defects (12/14) versus 77% of traumatic defects (13/17). Final union occurred in 100% of cases (14/14) and 82% (14/17), respectively. The improved outcome was attributed to the good vascularity of the soft tissue envelope surrounding the tumor resection defects. It is only after union takes place that resorption and susceptibility to stress fracture becomes apparent. Complete internal remodelling and restoration of mechanical strength requires 2 years. Shea et al15 and El-Gammalet al15found no difference in bony union in patients who underwent chemotherapy. Similar results were obtained in our study also.Two patients underwent radiotherapy. Among them, the one who had radioiodine therapy had graft site non-union. Being a solitary metastasis, we do not have any evidence that the radioiodine therapy had any influence on the graft union. Drawbacks:The patients were divided into the two groups, vascular and avascular fibula. Moreoverthe cohorts where retrospective. A randomised controlled study with longer follow up would perhaps yield clear and significant results.
CONCLUSION Fibula grafting after resection of tumors of the appendicular skeleton offers a satisfactory technique in reconstructing circumferential bony defects. It provides good bony union and functional outcome. Even though the postoperative functional outcome is significant, there is no significant difference between vascularized and avascular fibula in terms of bony union and graft hypertrophy.
REFERENCES
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home