Official Journals By StatPerson Publication
|
Table of Content - Volume 9 Issue 1 - January 2019
A study of clinical spetrum and out come of nephrotic syndrome in children
Jeetendra Kumar
Senior Resident, Dept. of Pediatrics, Vardhman Institute of Medical Sciences, Pawapuri, Bihar, INDIA. Email: jeetendrakr98@gmail.com
Abstract Background: Nephrotic syndrome is a common kidney disease across the globe and is an important chronic kidney disease in childhood. Nephrotic syndrome is a clinical syndrome characterized by massive loss proteins in urine called primarily albuminuria which leads to hypoalbuminemia. Objectives: The objectives of this study are to see clinical spectrum and outcome in children with nephrotic syndrome. Material and Methods: This was a prospective observational study done on 50 randomly selected children in pediatrics department, who were investigated to see clinical profile and outcome of nephrotic syndrome. Results: Male formed majority 66% of study. Swelling over body was most common symptom seen in all cases. All the cases showed pitting edema. 45% cases had first attack. Complete remission was seen in 32% cases while steroid dependence was seen in 10% cases. Conclusions: Overall clinical profile and laboratory findings were fitting to usual representation of nephrotic syndrome in children. Key Word: Nephrotic syndrome, Steroid resistance, Complete Remission
INTRODUCTION Nephrotic syndrome (NS) is the most common childhood kidney disease worldwide,1 Estimates on the annual incidence of nephrotic syndrome range from 2-7 per 100,000 children,2 and prevalence from 12-16 per 100,000. There is epidemiological evidence of a higher incidence of nephrotic syndrome in children from south Asia.3 Nephrotic syndrome (NS) is characterized by substantial loss of protein in the urine (primarily albuminuria), leading to hypoproteinemia (hypoalbuminemia) and its result, edema. Hyperlipidemia, hypercholesterolemia, and increased lipiduria are usually associated. Although not commonly thought of as part of the syndrome, hypertension, hematuria, and azotemia may also occur. NS is usually due to a glomerular disease and is currently categorized into primary and secondary forms.4,5 Most children with nephrotic syndrome have a form of primary or idiopathic nephrotic syndrome and the most common glomerular lesion is minimal change disease. The idiopathic nephrotic syndrome most commonly appears between the ages of 2-6 years of age, more common in boys and it is steroid sensitive in majority of cases[95%]. The cause of idiopathic nephrotic syndrome is unknown but evidence suggests that primary T cell disorder leading to glomerular podocyte dysfunction. 5 The clinical presentation of nephrotic syndrome vary widely from mild edema to severe cases presenting with complications important being life threatening infections and thromboembolic episodes. Nephrotic syndrome with significant glomerular lesion can have hypertension, renal insufficiency, and gross haematuria. Overall incidence of MCNS has been generally stable over past 3 decades. However incidence of FSGS seems to be increasing.5 Secondary nephrotic syndrome due to systemic causes include SLE, HSP, Amyloidosis, DM, HIV, Parvovirus B19, and Hepatitis B AND C virus infections.5,6 There is lack of studies on clinical profile of nephrotic syndrome in children in this areas. So we decided to do this study in order to assess clinical profile, complications and outcome in children with nephrotic syndrome. MATERIAL AND METHODS This was a prospective observational study conducted at pediatric department at Vardhman Institute of Medical Sciences 50 children who were diagnosed with nephrotic syndrome at Vardhman Institute of Medical Sciences in whom steroid treatment was not started yet were included for study purpose. Patients with first attack and relapse both were included in this study. Nephrotic syndrome was diagnosed based on the following criterias – 1) massive proteinuria > 40mg/m2/hr. or protein creatinine ratio >2-3:1 2) hypoalbuminemia <2.5gm/dl 3) generalised edema and lastly 4) hypercholesterolemia >200 mg/dl. Nephrotic syndrome secondary to systemic causes was not taken into consideration. Informed written consent was taken from the parents/guardians. Prestructured proforma was used for taking history, appropriate examination and investigations were done, investigations like complete blood count, peripheral smear, serum albumin, urine examination and culture etc were done in all the patients. Sulfosalycylic acid test was used for urine proteins, Esbach's albuminometer was used for protein creatinine ratio and 24 hours urine protein. BP, weight, intake and output chart, abdominal girth, urine for proteinuria were done daily on all patients. Patients were started treatment with steroids according to IAP protocol, along with fluid and salt restriction and their response was noted. Statistical analysis was done by standard descriptive statistics including chi-square test and calculating the p value. Institutional ethical committee approved this study.
RESULTS Table 1: Age and sex wise distribution of cases
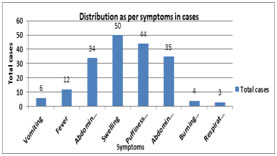
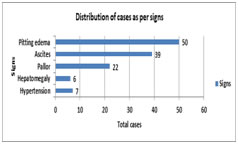
Diagram 1: Graphical representation of presenting symptoms in nephrotic syndrome Diagram 2: graphical representation of signs in nephrotic syndrome
Table 2: Laboratory profile of Nephrotic syndrome
Table 3: Outcome of nephrotic syndrome in cases
DISCUSSION In the present study children presented between the ages of 1-15 years with mean age at presentation being 8.3 years. Similar conclusion was drawn by Chahar OP et al,7 and Shastri NG et al.8 Nephrotic syndrome in younger age group is more likely is the chance of Minimal Change Nephrotic Syndrome/MCNS (Alhassan A et al9) In the present study, 68% of the cases belonged to 6-10 years age group followed by 1-5 years age group, which accounted for 24% of the nephrotic syndrome patients. Sahana KS et al10 concluded similar results with mean age of 7.4 yrs and most common age group of 6-12 yrs counting for 65% of cases. In this study 66% of the cases were male while as 34 % of cases were female with male to female ratio of 1.94:1, suggesting male dominance in study. In Safaei A et al11 there were 29 boys (66%) and 15 girls (34%), male:female ratio was 1.9/1; this was in accordance with our study. In other studies Ozkaya N et al12, Bircan Z et al13, Madani AB et al14, Siegal NJ et al15 this gender ratio ranged from 1.6 to 2.76/1. In this study 45% cases presented with first attack and 55% presented with one or more attacks previously which was not found to be statistically significant. Almost similar results was noted by Sahana KS et al10 with 36 % cases presented for the first time and 63% of patients had one or more episodes at the time of presentation which not statistically significant. In this study ascites was seen in 78% cases. Similar was seen with Sahana KS et al10 who concluded ascites in 63% of cases and in Safaei et al11 also had similar results. In this study swelling was most common symptom seen in all the patients which was in accordance with Sahana KS et al10 where as in a study done by Safaei A et al11, it was found to be 54.5%. other common symptoms were abdominal distention 70% and pain 68%. Other symptoms noted by various studies Chowdhary EUA et al16 and Safaei A et al11 include anorexia, lethargy, abdominal pain and diarrhoea. In this study hypertension was seen in 14% cases. While in a study by Struss.J et al17 hypertension was found to be present in 20.7% of cases with MCNS and in 25.7% of cases with other histological types. Nelson et al also concluded similar 10% findings in their study. In this study hemoglobin ranged from 8.1-11.2 gm/dl and 60% cases had anemia Sahana KS et al10 observed anemia in 74% while in Anochie et al18 half the cases presented with anemia. Iron deficiency anaemia in nephrotic syndrome is attributed to loss of transferrin in the urine. serum albumin was in normal range 1.2-2.2 gm/dl and mean±SD was1.8±0.45. Similar observations made Hiraoka M et al.19 In this study steroid resistance was seen in 6% cases which was in similarity with Sahana KS et al10 with 3% cases showing initial resistance and Banh TH et al20 reported 2.5% cases with initial resistance. While in Kim JS et al21 it was found to be about 15 %, which was higher than our study which may be due to larger sample size. Banh TH et al20 reported 22% remission rate while in this study it was 32% which was almost similar. Steroid dependence was seen in 10% cases in this study which was in accordance with Banh TH et al20 study 9%. There was one death seen among all cases while in with Sahana KS et al10 there was no any deaths.
CONCLUSION In our study clinical and laboratory findings were in similarity with usual nephrotic syndrome in children. There was no any significant difference in pattern of nephrotic syndrome and response to treatment from other studies.
REFERENCES
|
|
 Home
Home