Official Journals By StatPerson Publication
|
Table of Content Volume 11 Issue 3 - September 2019
Cutaneous adverse drug reactions in Telangana population
R Sreedhar1*, R Aruna2
1Associate Professor, Department of Pharmacology, Kamineni Institute of Medical Sciences, Narketpally, Nalgonda (dist) – Telangana, Andhra Pradesh, INDIA. 2Associate professor, Department of Pathology, Chalmeda Ananda Rao Institute of Medical Sciences, Karimnagr Telangana, Andhra Pradesh, INDIA. Email: drsreedharkrnr@gmail.com
Abstract Background: in everyday clinical practice many of the doctors come across many instances of cutaneous adverse drug reaction CADR. although such CADR are common, comprehensive information regarding their incidence severity, types and ultimate health effects are not reported or neglected. In the modern world many new drugs are used, and new drugs CADR are neglected or un reported Methods: study was conducted over the period of 2 years in the outpatient and inpatient departments. Drug reactions, severity was confirmed by Naranjo Adverse Drug reaction probability scale, RegiScar criteria was used for diagnosing DRESS syndrome CBP, RBS, Urine. Sr. creatinine, liver enzymes were studied. In severe cases LFT, serum electrolytes and chest x-ray was also studied. Results: The various symptoms of cutaneous drug reaction were classified with percentage and range of incubation period was noted. The drugs prescribed and common symptoms, number of patients sever symptoms, were also classified with percentage. Conclusion: This empirical study high lights the common and severe adverse drug reactions and drugs which cause adverse reactions so that early withdrawal of drug and improves the outcome. Patients also informed that to which drug they are sensitive and sensitive drug list should be kept with them. Key Word: Naranjo Scale, Regi SCAR criteria, DRESS syndrome, causative Drugs, Stevens- Johnson syndrome.
INTRODUCTION Cutaneous drug reactions are common among the adverse drug reactions; they account for patients suffering hospitalization and economic burden and sometime be fatal. The common symptoms of the reactions are skin rash. Urticaria, fixed drug eruption, angioedema and contact dermatitis. Serious cutaneous drug reactions like stevens-Johnson syndrome, toxic epidermal necrolysis (TEN), drug reactions with eosinophilia and systemic symptoms (DRESS) and acute generalized exanthematous prustusis (AGED)1. The common offending drugs are antimicrobials, non-steroidal anti inflammatory drugs (NSAID), antiepileptic drugs and anti-gout drugs2. The cutaneous drug reaction pattern and causative drugs may very with prescribing habit and level of health care3 Majority of the drug reactions are diagnosed clinically. Recognition of the offending drugs enables early withdrawal of drug and improved out come. Hence attempt was made to study the cutaneous adverse drug reactions and the drug which causes the reaction.
MATERIAL AND METHODS A prospective observational, non randomized hospital based study is Done in About 180 adult patients aged between 19 to 65 years regularly visiting kamineni medical college Hospital Narketpally- Nalgonda (Dist), Telangana Inclusion criteria: The patients having cutaneous drug reactions of both out patients and inpatients of dermatology department were included in the study. Exclusions criteria: The patients with generalized purities with skin lesions, self-medicated patients and who don’t recall the names of drugs HIV and psychotic patients were excluded from study
METHODS the detail history of drugs taken, route of administration careful clinical examination was done and significant finding were noted. The severity of cutaneous drug reactions were confirmed by Naranjo Adverse drug reaction probability scale4. Culprit drug was determined based on the chronology from the introduction of the drug to the onset of symptoms. If more than one drug was thought to be responsible, then most likely offending agent was noted and withdrawn. A Regiscar criteria was used for diagnosing DRESS syndrome 5. (Drug reaction with esinophilia and systemic systems syndrome) to evaluate prognosis of TEN (Toxic Epidermal Necrolysis) was done by SCORTEN criteria 6. Blood count (CBP), urine analysis, RBS, liver enzymes blood urea and serum creatinine were carried out in all patients. In severe cases, LFT, serum electrolytes, chest-x-ray were also done. Duration of study was about 2 years. This research paper was approved by ethical committee of Kamineni medical college Narketpally. Nalgonda(dist)-Telangana Statistical analysis: various symptoms of the drug reactions were classified with percentage and the range of incubation period was also studied. The causative drugs were classified with their common and severe symptoms with percentage are studied. The ratio of the male and female were 1:2
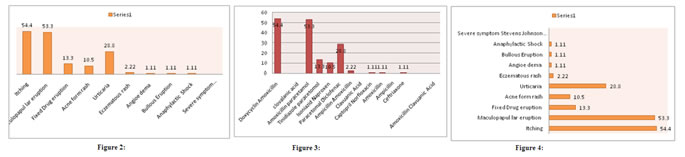
OBSERVATION AND RESULTS Table 1: The symptoms of cutaneous drug reactions. With their incubation period 98 (54.4%).,Itching -96(53.3%), redness the range of incubation period was 30 minutes to 3 weeks 52(28.8%) rash, incubation range was total 4 week 19(10.5%) A incubation range was lo dy sto 4 week, 24(13.3) eruptions incubation period was 1 day to 7 weeks 11(6.1% hyper pigmentation. Incubation range was 4 to 6 weeks. 4 (2.2%) Nail discoloration, 3(1.6%) was hirsutism. Table 1: Symptoms of cutaneous adverse drug Reactions with their incubation period
No of Patients -180 Table 2: (a) Common symptoms:- 98 (54.4%) patients had pruritis causative drugs were Doxycycline, Amoxicillin, clavulanic acid. 96 (53.3%) had muculo papular eruption, the causative drugs were Amoxicillin Paracetamol. 24(13.3%) had fixed drug eruption. The causative drugs were Tinidazole, Paracetamol 19(10.5%) had Acneiform rash, the causative drugs were Isoniziad, Naproxen, 52(28.8%) had urticaria the causative drugs were paracetamol, diclofenac, 4 (2.22) had Eczematous rash, the causative, drugs were Ampicillin, Amoxicillin clavulanic Acid 4(2.22%) had Angioedema, the causative drugs were captopril, norfloxacin, 2(1.11%) had bullous eruption 2(1.11%) had Anaphylactic shock, the causative drug was ceftriaxozone (b) The severe symptoms-3 (1.66%) had SJS, the causative drugs were Teribinafine, Neviparine, ciprofloxacin, Amoxicillin, clavulanic acid.
Table 2: Common symptoms and list of drugs that causing cutaneous adverse drug reactions
No of Patients –180 Figure 2: Common Symptoms; Figure 3: Causative Drugs
DISCUSSION In the present study of cutaneous adverse drug reactions in Telangana population. The symptoms of cutaneous drug reactions were 98(54.4%) had Itching 96 (53.3%) had redness incubation period was 30 minutes to 3 weeks , 52 (28.8%) had rash the incubation period was day to weeks, 19 (10.5%) Acne the incubation period was 10 days to 4 weeks 24(13.3%) eruptions incubation period was 1 day to 7 weeks, 11 (6.1%) had hyper pigmentation, incubation range was 1 to 4 weeks 9(5%) hair loss incubation range was 4 to 6 weeks, 4(2.2%) had discoloration of Nail 3(1.6%) had Hirsutism (Table-1). Classification of Drugs causing coetaneous drug reactions-(a) common symptoms 98(54.4%) had pruritis, the causative drugs were doxycycline, Amoxycillin, clavulanic acid, 96(53.3%) had maculopupular eruption the causative drug were Amoxycillin, Paracetamol, 19(10.5%) had fixed drug eruption the causative drugs were Isoniazid, Naproxen, 52(28.8%) had urticaria, the causative drugs were paracetamol, diclofenac, 4(2.22%) had Eczematous rash the causative drugs were Ampicillin Amoxicillin clavulanic Acid 4(2.22%) had Angioederma the causative drugs were captopril, Norfloxacin, 2(1.11%) had Anaphylactic shock, the causative drugs were ceftriaxone (b). The severe symptoms 3(1.66%) had SJS. The causative drugs were Terbinafine, Nevirapine, ciprofloxacine, Amoxicillin, clavulanic acid (Table-2). These findings were more or less in agreement with previous studies7,8,9. Total number of causative drugs were (average one drug per case). The major suspect groups were antimicrobials (45.4%) NSAID (20.8%) antiepiletics (14.5%) and corticosteroids (3.8%)10. Anti-retroviral group of drugs like efavirenz, nevirapine, Zidovudine Tenofovir, lamivudine, stavudine, Abacavir were next common drugs having cutaneous drug reactions from mild to severe Stevens- Johnson reaction. another groups of drugs which cause cutaneous, reactions are anti-tubercular drugs, especially first line of anti-TB drugs like (Rifampicin, isoniazid, pyrazinamide, ethambutol) and anti-epileptic drugs like phenytoin carbamazepine, were also observed11. It was reported that almost2/3rd of cutaneous drug reactions were preventable. It requires cautious interpretation as preventability assessment was based on small sample size12. Moreover known treatment for adverse drug reaction considered as probably preventable
SUMMARY AND CONCLUSION The present study of cutaneous adverse drug reactions is quite helpful not only for dermatologist but also to the clinicians because there is no gold standard investigation is available for diagnosis of cutaneous adverse drug reactions but taking a proper history, such as duration of drug intake, reaction time, response of drug eruption to withdrawl of the suspected drug. Early identification of cutaneous drug reactions can reduce the morbidity and mortality. Most important is previous history drug reaction to particular drug is to be avoided. This study further demands to educate the patients to avoid self administration of drugs, moreover this study also warrants immunological, nutritional, patho-phyislogical, microbiological, pharmacological, genetic study because exact mechanism of cutaneous adverse drug reactions is still not-clear.
REFERENCES
|
|
 Home
Home