|
Table of Content Volume 16 Issue 3 - December 2020
Pharmacological evaluation of anticonvulsant effect of carvedilol on pentylenetetrazole induced convulsions in wistar albino rats
Biacin Babu1*, Madhavrao C2
1Assistant Professor, Department of Pharmacology, Malabar Medical College Hospital and Research Centre, Calicut, Kerala, INDIA. 2Associate Professor, Department of Pharmacology, All India Institute of Medical Sciences, Mangalagiri, Andhra Pradesh, INDIA. Email: drbiacin84@gmail.com
Abstract Background: Epilepsy is a chronic disorder due to the imbalance of inhibitory and excitatory neurotransmitters in the brain. The present study aimed to evaluate the anticonvulsant effect of Carvedilol on pentylenetetrazole induced convulsions in Wistar Albino rats. Materials and Methods: Total of 30 rats were divided into 5 groups each of 6 rats. G-I Normal Saline (0.9%/PO), G-II Sodium valproate (400 mg/kg/BW/i.p),G-III: Carvedilol (1 mg/kg/BW/PO), G-IV: Carvedilol (2 mg/kg/BW/PO) and G-V: Carvedilol (4 mg/kg/BW/PO). All groups were given PTZ (70mg/kg/BW/sc) after 30 min of administration drugs. Onset, duration, number and scores of seizures were recorded. SPSS (16.0) version used for analysis. Results: Group-I showed significant difference compared to all other groups. Group-II showed significant protection compared to others groups. High dose of test drug showed better effect compared to low doses. Similar results were observed in all the observations. Conclusion: Decreased adrenergic neurotransmitter effect can prevent the development of seizures. Carvedilol is adrenergic blocker showed anticonvulsant at high dose compared to low dose. Key Words: Anticonvulsant, carvedilol, calcium channel blocker, epilepsy, Pentylenetetrazole, sodium valproate.
INTRODUCTION Epilepsy is the second most common neurological disorder after stroke. It affects between 0.5-1% of the population world wide1.The prevalence rates of epilepsy in India are similar to those of developed nations. However, the large treatment gap is a major challenge to our public health system. A wide variety of modalities for arresting and controlling seizures have been employed, for example yoga, drugs, surgery, etc. But drug therapy remains the mainstay in treatment ofepilepsy2.Currently, there is no single drug of choice in treating all types of seizures and no drug that has substantially lowered the percentage of patients who are drug resistant. The current therapy of epilepsy with modern antiepileptic drugs is associated with side effects, dose related and chronic toxicity and approximately30% of patients continue to have seizures with current antiepileptic drug therapy.3,4 The use of these antiepileptic drugs in females of reproductive age group has much greater importance due to various issues related to interactions with oral contraceptives, potential for teratogenicity causing neural tube defects and also effects on vitamin K metabolism5.Many measures are taken up by physicians in order to overcome various problems associated with the use of antiepileptic drugs. The degree of success of use of these drugs depends on the seizure type, cause and patient compliance. The physician starts the drug therapy using a single drug for minimizing toxicity. If the seizures are not controlled with the first line drugs at normal therapeutic plasma concentrations, then 2nd line drugs can be started concurrently with the first line drugs. It is ideal to measure the plasma drug concentrations once multiple drug therapy is initiated, treatment failure is seen or when toxic effects appear. Carvedilol is a third generation beta adrenoceptor antagonist and has a unique pharmacological profile. It has α1, β1 and β2 blocking property and is currently indicated for the treatment of mild to moderate congestive heart failure6.It is highly lipophilic and thus extensively distributed to extravascular tissues.6 It has been shown to possess antioxidant, antiplatelet and anti-inflammatory properties that attribute to its neuroprotective effects7,8.It has a plasma half life of 7 to 10 hours with a plasma protein binding of 98%9.The drug is better tolerated as it is not expected to be toxic following ingestion10.Pentylenetetrazole (PTZ) is a drug commonly used to induce the seizures. It induces the seizures in animals like humans. According to review literature the present study aimed to evaluate the anticonvulsant effect of Carvedilol on pentylenetetrazole induced convulsions in Wistar Albino rats.
MATERIALS AND METHODS Study settings and period This study was done in Department of Pharmacology, Sree Mookambika Institute of Medical Sciences, Kulasekharam, Tamil Nadu. The study protocol was approved by Institutional Animal Ethics Committee (IAEC). Animals Healthy adult male Wistar albino rats weighing 150-250 g were obtained from the central animal house of the institute. Total 60 male Wistar albino rats were used for this study purpose. Female Wistar albino rats were not included in this study as they are more susceptible to seizures and any chance of error in data analysis was eliminated. Wistar albino rats were procured from the central animal house. All the animals used for the experiment were maintained on standard laboratory food pellets and water ad libitum. The animals were transferred to the experimental room of central animal house for a period of 5-6 hours/day for acclimatization. 3 Wistar albino rats were placed in a single plastic perspex cage at room temperature (27±20C), humidity70-80%, maintaining 12±1 hour light and dark cycle42.The experimental animals were fasted for 8 hours prior to the study with free access only to water. All the experiments were performed in the experiment lab of the central animal house at the same time of the day (between 9:00 AM and 1:00 PM) to minimize influences of circadian rhythm on seizure susceptibility.
Groups Group-I: Normal Saline (0.9%/PO); Group-II: Sodium valproate (400 mg/kg/BW/i.p); Group-III: Carvedilol (1 mg/kg/BW/PO); Group-IV: Carvedilol (2 mg/kg/BW/PO); Group-V: Carvedilol (4 mg/kg/BW/PO). Pentylenetetrazole Induced Convulsions Healthy adult male Wistar albino rats weighing 150-250 g were chosen. The test drug carvedilol (in doses 1, 2, 4 mg/kg body weight orally) and standard drug sodium valproate (400 mg/kg body weight intraperitoneally) was administered to Wistar albinorats. After 30 minutes of administration of the drugs, PTZ was given in a dose of 70mg/kg body weight by subcutaneous route. Each animal was placed into an individual plastic cage for observation lasting for 1 hour. Seizures and tonic clonic convulsions were recorded and scored as shown below. Scores Description12 0: No behavioral changes like grooming, sniffing etc 1: Isolated Myoclonic jerks, ear and facial twitching 2: Atypical minimal seizures, convulsive wave through the body 3: Fully developed minimal seizures, clonus of head muscles andforelimb, righting reflex present 4: Major seizures (Generalized without tonic phase) 5: Generalized tonic-clonic seizures beginning with running Statistical analysis The data was expressed in mean and standard deviation. Statistical Package for Social Sciences (SPSS 16.0) version used for analysis. One way ANOVA (Post hoc) followed by Dunnet t test applied to find the statistical significant between the groups. p value less than 0.05 (p<0.05) considered statically significant at 95% confidence interval.
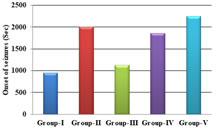
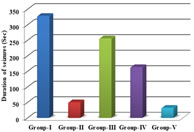
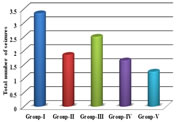
RESULTS The study showed statistical significant increase in onset of seizure time in group-II[p<0.01], group-IV [p<0.05] and group-V [p<0.001] when compared to group-I. There was also significant increase in onset of seizure time in group-V when compared to group-III [p<0.01]. There was also increase in onset of seizure time ingroup-III when compared to group-I, however it was not found statistically significant[p>0.05]. There was no statistical significant difference was found between group-II when compared to group-IV and group-V [p>0.05]. There was increase in onset of seizure time in group-V when compared to group-II, but it was not found statistically significant [p>0.05]. The study showed statistical significant decline in duration of seizure time in group-II [p<0.01], group-IV [p<0.01] and group-V [p<0.001] when compared to group-I. There was also significant decline in duration of seizure time in group-V when compared to group-III [p<0.001] and group-IV [p<0.05]. There was also decline induration of seizure time in group-III when compared to group-I, however it was not found statistically significant [p>0.05]. There was no difference in statistical significance between group-II when compared to group-IV and group-V [p>0.05]. There was decline in duration of seizure time in group-V when compared to group-II,but it was not found statistically significant [p>0.05]. The study showed statistical significant reduction in number of seizures in 1 hour ingroup-II [p<0.01] and group-V [p<0.01] when compared to group-I. There was also significant reduction in number of seizures in 1 hour in group-V when compared to group-III [p<0.05]. There was also reduction in number of seizures in 1 hour in group-III and group-IV when compared to group-I, however it was not found statistically significant [p>0.05]. There was no statistical significant difference was found between group-II when compared to group-IV and group-V [p>0.05]. The study showed statistically significant decrease in scores of seizures in group-II[p<0.01] and group-V [p<0.001] when compared to group-I. There was also significant decrease in scores of seizures in group-V when compared to group-III[p<0.05]. There was also decrease in scores of seizures in group-III and group-IV when compared to group-I, however it was not found statistically significant [p>0.05].There was no statistically significant difference found between group-II when compared to group-III, group-IV and group-V [p>0.05].
Table 1: Comparison of onset, duration and total number of seizures between the groups
(*p<0.05 significant compared group-I with other groups, #p<0.05 significant compared group-II with other groups, $p<0.05 significant compared group-III with other groups, ǁp<0.05 significant compared group-IV with other groups)
Graph 1: Comparison of onset of seizures between the groups; Graph 2: Comparison of duration of seizures between the groups; Graph 3: Comparison of total number of seizures between the groups
DISCUSSION Our study showed dose dependent increase in onset of seizure time in carvedilol groups in pentylenetetrazole model. The increase in onset of seizure time was highest with carvedilol (4 mg/Kg) body weight and lowest with carvedilol (1 mg/Kg) bodyweight. It can be compared with the study shown by Goel et.al13who has done the study in mice with carvedilol (1.25, 2.5 and 5mg/kg) body weight where a significant(p<0.01) prolongation of the latency of clonic jerks was shown when compared with the control. In our study, the increase in onset of seizure time was shown in a dose dependent manner with pentylenetetrazole model using carvedilol (4 mg/Kg BW) and was comparable to that of standard drug sodium valproate. Carvedilol exhibited decline in the duration of seizure time in dose dependent manner in pentylenetetrazole model. The decline was highest with carvedilol (4mg/kg/BW). The decline in duration of seizure time in dose dependent manner in pentylenetetrazole model in carvedilol (2 mg/Kg/BW) and (4 mg/Kg/ BW) was comparable to sodium valproate. This is in comparison to the study done by Kharashi et al.,15 where carvedilol (3mg/kg/BW) in pre ischemic injury (repeated dose injection for 4 days) or post ischemic injury (single dose injection)caused a significant decrease in duration of seizures. Carvedilol also showed significant decline in number of seizures in 1 hour in carvedilol (2mg/kg/BW) and (4mg/kg/BW) when compared to the control group. The decline in number of seizures in 1 hour in carvedilol groups was dose dependent with highest decline shown in (4 mg/Kg/BW) carvedilol and least with (1mg/Kg/BW).The decline in number of seizures in 1 hour in a dose dependent manner was shown in pentylenetetrazole model with carvedilol (2 mg/Kg/BW) and (4 mg/Kg/BW) and was comparable to standard drug sodium valproate. Our study showed significant decline in scores of seizures in pentylenetetrazole model with carvedilol (2mg/kg/BW) and (4mg/kg/BW) when compared to the control. The decline in scores of seizures in carvedilol treated groups was dose dependent with highest decline in carvedilol (4 mg/Kg/BW) and least decline in carvedilol (1mg/Kg/BW). The decline in scores of seizures in dose dependent manner in pentylenetetrazole model in carvedilol (1 mg/Kg/BW), carvedilol (2 mg/Kg/BW) and (4mg/Kg/BW) was comparable to standard drug sodium valproate. Carvedilol has good lipid solubility15-19 and hence crosses the blood brain barrier effectively. So adequate concentration of carvedilol in the brain can be one of the factors in controlling the seizures. In a study done by Louis et.al.,20it was shown that the centrally present beta adrenoceptors when blocked by non selective betablockers both propranolol and timolol (in low doses) produced anticonvulsant action, but practolol, a selective beta-1antagonist, did not show any anticonvulsant action. This indicated that the anticonvulsant action of propranolol and timolol was due to the blockade of beta-2receptors. It was also demonstrated that local anesthetics have a protective effect against convulsions induced by PTZ model. The membrane stabilization action of propranolol in high doses (>1mg/kg/BW in rats) was postulated to be the likely mechanism of action in PTZ induced convulsions in rats. Carvedilol has a membrane stabilizing action may be due to the inactivation of sodium channels and likely the mechanism of its anticonvulsant action.
CONCLUSION Adrenergic blocker carvedilol showed significant anticonvulsant effect. Further studies required to evaluate the required dose with less adverse effects. There is requirement of molecular studies to find the mechanism of action.
REFERENCES
|
|
 Home
Home