Official Journals By StatPerson Publication
|
Table of Content - Volume 6 Issue 3 - June 2018
Effect of depression treatment on asthma patients
Samyuktha G1, Ajaykumar Dhage2*
1Consultant, Psychiatrist, Ameya Healthcare HSR layout Bangalore, Karnataka, INDIA. 2Assistant Professor, Department of Psychiatry, M. R. Medical College, Gulbarga, Karnataka, INDIA. Email: veda26@gmail.com
Abstract Background: Many asthmatics develop significant psychiatric reactions to some of the drugs used in its treatment. Depressed affect is a common reaction to the illness and may make the condition more difficult for the primary care physician to manage. Aim: To study the effect of depression treatment on asthma patients. Material and Methods: Seventy asthmatic patients between the age group 18-65 years were studied. Patient Health Questionnaire (PHQ9) was used to screen the subjects for depression. Appropriate treatment was given for depression and asthma as per standard guidelines. Asthma control was assessed using Asthma Control Questionnaire (ACQ) which includes Forced Expiratory Volume (FEV1).These scales were administered at baseline, 3rd and 6th months post-treatment. Results: Depression had no significant correlation with asthma control at baseline. At 3rd month follow up, along with decrease in depressive scores (PHQ9), there was a significant decrease in ACQ scores, implying improvement in asthma control. There was no significant decrease in FEV1. At 6-months follow up, with reduction in depression scores, there was significant reduction (improvement) in both objective and composite measures of asthma control. Conclusion: Treatment of depression among asthmatics improves the asthma control and quality of life to a level similar to those without depression. The results indicate the need to screen all asthma patients for depression. Key Words: Asthma, depression, treatment, Patient Health Questionnaire, Forced Expiratory Volume, Asthma Control Questionnaire.
INTRODUCTION Asthma or hyperreactive airways disease usually is a chronic illness that may be mild or severe enough to be life-threatening. Many asthmatics develop significant psychiatric reactions to some of the drugs used in its treatment. Depressed affect is a common reaction to the illness and may make the condition more difficult for the primary care physician to manage1,2 Depressed patients tend to have low energy levels, and feelings of hopelessness, helplessness, and guilt, and so they often do not attend to the warning symptoms of an impending asthmatic attack. Since major depression frequently interferes with optimal medical management, it is important to consider and actively treat depression in asthmatics. The present study was carried out to study the effect of depression treatment on asthma patients.
MATERIAL AND METHODS This prospective comparative study was carried out at Department of Respiratory Medicine, in a tertiary care health center in Puducherry, India. A total of 70 patients between 18-64 years with respiratory symptoms suggestive of airway disease registered at were assessed using spirometry. Inclusion Criteria: Patients aged 18-64 years, registering at the Department of Respiratory Medicine for the first time, with a diagnosis of asthma subsequently confirmed through spirometry and attending follow-up, registered not earlier than 3 months prior to date of current visit, already diagnosed as asthma using spirometric criteria were included. A written informed consent was obtained from each patient. Exclusion Criteria: Patients with other co-morbid general medical conditions (hypertension, diabetes mellitus, COPD, etc.), who have received psychiatric treatment elsewhere and are on treatment currently, who cannot perform spirometry, smokers and severely ill patients unable to co-operate for psychiatric evaluation were excluded from the study. Methodology: Diagnosis made using Patient Health Questionnaire(PHQ-9) was verified by a consultant psychiatrist, using WHO ICD-10 Diagnostic Criteria for Research, through a formal psychiatric interview.PHQ-9 was used only to screen depression. Baseline ratings of PHQ were obtained from all asthmatic subjects. Group 1: Asthmatic subjects who received a significant score on PHQ-9 (asthmatics with depression) were classified as Group 1. Group 2: Asthmatic subjects who did not receive a significant score on PHQ-9 (asthmatics without depression) were classified as Group 2. Spirometric measurements that were obtained from newly registered cases for the purpose of confirming the diagnosis of asthma were treated as baseline values. Spirometric values of follow-up cases of asthma were recorded at the time of enrolment into the study, and were treated as baseline values for the purpose of this study, irrespective of prior spirometric values. Spirometric Criterion -Forced Expiratory volume in one minute (FEV1): This method was used to measure airway limitation and reversibility to establish diagnosis of asthma. FEV1 values were monitored and it was a part of ACQ measured by clinician (objective finding). ACQ (Asthma Control Questionnaire): The ACQ has been validated to measure the adequacy of asthma management. Responses are given on a 7-point scale and the overall score is the mean of responses. The scale consists of 5 symptoms (shortness of breath, wheezing, waking dyspnea, nocturnal dyspnea, activity limitations) bronchodilator use and measurement of FEV1completed by the clinician. The ACQ has strong evaluative and discriminative properties and can be used with confidence to measure asthma control.3 Treatment and follow-up
Statistical Analysis: Data analysis was performed using the Statistical Package for Social Sciences (SPSS v 13.0), EPIINFO v. 6.0d and Microsoft Excel software. RESULTS A total of 70 adult asthma patients participated in the study. Their median age was 33 years (range: 18-65). Of the participants, 39% were male and 61% female with corresponding median (mean) ages were 30 (35.9) and 35 (37.3) years respectively. Both the median (95% confidence intervals about median overlap for genders) and mean (Student’s t-test for independent samples, P=0.69) ages do not differ significantly between genders. The median (mean) age of the patients in Groups 1 and 2 were 42 (40.4) and 30 (32.9) years respectively (Figure 2). Both the median (95% CIs about median do not overlap for the Groups) and mean ages of the patients differed significantly (Student’s t-test for independent samples, P=0.02). The percentages of male and female patients in Group 1 (n=32) were 25 and 75% and the corresponding figures in Group 2 (n=30) were 53 and 47%. The gender distributions differed significantly between Groups 1 and 2 (χ2=5.2, P=0.02). Associations of PHQ9 score with FEV1 and ACQ scores: While at baseline (r=0.3041, P=0.09) and 3 months post-treatment (r=0.3179, P=0.07) no significant correlations were observed between PHQ9 score and FEV1 score, at 6-months post-treatment significant positive correlation (r=0.6044, P=0.0007) was observed between the two variables. Significant positive associations were observed between PHQ9 and ACQ scores both at 3 months (r=0.5625, P=0.0008) and 6 months (r=0.8034, P<0.0001) post-treatment. However, at baseline no significant association was observed (r=0.1627, P=0.37). It was observed that of out the 40 asthmatic patients with depression, only one patient had complete remission (PHQ9 score-0), eight had minimal depression (PHQ9 score- 1 to 3) and one patient did not respond to the treatment. Changes in mean scores: The PHQ9 score declines from 13.9 at baseline to 8.6 and 4.6 respectively at 3 and 6 months post-treatment. The corresponding scores at baseline, 3 and 6 months post-treatment were 1.9, 1.1 and 0.7 for FEV1 and
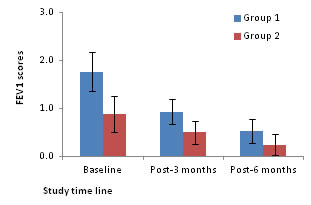
Figure 1: FEV1 scores as a function of asthmatic patients
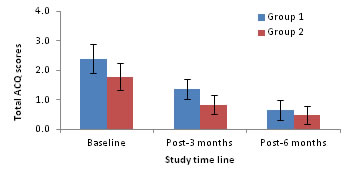
18.2, 10.8 and 5.8 for ACQ. Statistical analysis revealed that while the decline in mean scores from baseline to 3 and 6-months post-treatment were significant for both PHQ9 and ACQ (One way ANOVA followed by post-hoc test based on LSD, P<0.05 for both comparisons), the decline in FEV1 score was significant only between baseline and 3 months-post-treatment (One way ANOVA followed by post-hoc test based on LSD, P<0.05).Further decline in FEV1 score from 3 months to 6 months post-treatment was not significant (P>0.05). Extent of improvement in depression, asthma control and quality of life: A significant positive association was found between extent of improvement in PHQ9 score (r=0.61, P=0.001) with ACQ score. Asthmatic patients without depression Associations of PHQ9 score with FEV1and ACQ scores The FEV1 score had no significant association with PHQ9 score at baseline, 3 and 6-months post-treatment. Whereas, PHQ9 score had significant positive and negative associations with the scores of ACQ (r=0.5779, P=0.0008) at 3 months post-treatment. However, these variables did not significantly associate at baseline or 6-months post-treatment. Changes in mean scores: The mean scores of PHQ9 and ACQ significantly declined at 3 and 6 months post-treatment when compared to their corresponding scores at baseline (one way ANOVA followed by post-hoc test based on LSD, P<0.05 for all comparisons). Comparison between groups: Figure 1 compares the FEV1 scores at baseline, 3 and 6 months post-treatment. The FEV1 scores at baseline, 3 and 6 months post-treatment are 1.8, 0.9 and 0.5 for Group1 and 0.9, 0.5 and 0.2 for Group 2 respectively. The FEV1 scores for Group 1 are always higher than that of Group 2 but for both the groups the scores decline with duration of treatment. Comparison of the scores between Groups indicated that the Group 1 had significantly higher scores at baseline (1.8 vs 0.9) and at 3 months post-treatment (0.9 vs 0.5) but not at 6 months post-treatment (0.5 vs 0.2; P=0.10) (Two way ANOVA). The total ACQ scores for the two groups (with and without depression) are compared at baseline, 3 and 6-months post-treatment in Figure 2. The total ACQ scores are 2.4 and 1.79 at baseline, 1.38 and 0.85 at 3 months post-treatment and 0.75 and 0.49 at 6 months post-treatment for Groups 1 and 2 respectively. The ACQ scores indicate that at baseline both groups are comparable with uncontrolled asthma (ACQ score >1.5). As a result of treatment, the ACQ scores declined for both the groups to reach the state from uncontrolled to inadequate (ACQ score: 0.75-1.5) and well controlled (ACQ score <0.75) states at 3 and 6 months post-treatment respectively. Analysis on group differences in total scores on the ACQ revealed that the groups did not differ significantly at baseline (Two way ANOVA, P>0.05) and 6 months post-treatment but differed significantly at 3 months post-treatment (P=0.03), indicating that patients with depression (1.38) had significantly higher total ACQ scores at 3 months post-treatment compared to patients without depression (0.85). However, at 6 months post-treatment the ACQ scores were comparable between patients with and without depression indicating impact of treatment on Group 1.
Figure 2: Total ACQ scores (mean) as a function of asthmatic patients
DISCUSSION In this study it was found that, depression improved over 6 months duration with treatment. Objective scores (FEV1) for both the groups declined with duration of treatment. The asthma control parameters (composite score) for both the groups indicate that at baseline both groups had uncontrolled asthma (ACQ score >1.5). As a result of treatment, the ACQ scores declined for both the groups to reach the state from uncontrolled to inadequately controlled (ACQ score: 0.75-1.5) and well controlled (ACQ score <0.75) states at 3 and 6 months post-treatment respectively. However, at 6 months post-treatment the ACQ scores were comparable between patients with and without depression indicating impact of treatment on asthmatics with depression. Asthma quality of life scores improved as a result of treatment. The quality of life for asthmatic patients with depression was poor compared to asthmatic patients without depression. However, at 6 months follow up quality of life of asthmatics with depression was almost similar to asthmatics without depression, implying that treatment of depression had a favorable impact on asthma quality of life and FEV1 scores. Two studies by Brown et al30;58 reported a reduction in depressive symptoms after treatment with Citalopram and Bupropion, which was associated with improvement in asthma control and quality of life. Treatment of depression among asthmatics improves the asthma control and quality of life to a level similar to those without depression.
CONCLUSION Treatment of depression among asthmatics improves the asthma control and quality of life to a level similar to those without depression. The results indicate the need to screen all asthma patients for depression and treat them appropriately to improve their quality of life and asthma control.
REFERENCES
|
|
 Home
Home