Official Journals By StatPerson Publication
|
Table of Content - Volume 10 Issue 2 -May 2019
A comparative study of Dexmedetomidine versus midazolam with fentanyl for monitored anaesthesia care in tympanoplasty under local anaesthesia
Jigisha Badheka1, Pratik M Doshi2*, Peram Shrividhya3, Jaykishan Gol4, Vandana Parmar5
1,2Associate Professor, 3Senior Resident, 4Junior Resident, 5Professor & HOD, Department of Anaesthesia, PDU Government Medical College, Rajkot, Gujarat, INDIA.
Abstract Background and Aims: Monitored anaesthesia care (MAC) is a procedure in which the local anaesthesia (LA) and sedation provided using different drugs. We conducted this comparative study to see the safety and efficacy of Dexmedetomidine and midazolam with fentanyl for tympanoplasty under MAC. Methods: Fifty patients of age between 18 to 60 years of either sex posted for tympanoplasty under MAC were randomly allocated into two groups. Group D (n = 25) patient received intravenous (IV) dexmedetomidine 1 µg/ kg as bolus followed by an infusion 0.2 µg/kg/h. Group MF (n =25) patient received IV midazolam 0.05 mg / kg plus fentanyl 1.5µg/ kg as bolus followed by 0.2 ml/kg/h normal saline as an infusion. Sedation was titrated according to Ramsay Sedation score of 3. Rescue doses of midazolam 0.01mg/kg IV as sedation and fentanyl 1µg/ kg IV as analgesic was given when required, maximum 3 doses allowed. Patient’s oxygen saturation, hemodynamics, and need for intraoperative rescue sedation/analgesia were assessed as primary outcome. Surgeon satisfaction score were assessed as secondary outcome. The data were analyzed by Chi-square and unpaired t-test. Result: Number of rescue analgesia /sedation/infiltration/ was less in dexemeditomidine group (2/2/4) compared to group MF (14/14/20). Surgeon satisfaction score was higher in group D than group MF (P=0.001). Haemodynamically patients were stable in both the groups. Conclusion: Compared to midazolam with fentanyl, dexmedetomidine is a better alternative as it is associated with good haemodynamic control, without respiratory depression, lower pain scores and greater surgeon satisfaction. Key Word: Tympanoplasty, Local anaesthesia, Sedation, Dexmedetomidine, Midazolam with Fentanyl.
INTRODUCTION Middle Ear Surgeries (MESs) can be performed under general anaesthesia or Monitored Anaesthesia Care (MAC). It is defined as a conscious sedation, with conserved answering to calling and preserved spontaneous breathing. During MAC patients undergo local anaesthesia (LA) together with sedation and analgesia. The main advantages of MAC is patient satisfaction and early discharge.1 Many advantages has been reported with the LA, like early recovery, less post-operative pain, cost effective and surgeons ability to test hearing during surgery. The patients of MESs under LA faced most common discomforts like noise during surgery, anxiety, dizziness, backache, claustrophobia and ear ache.2 To reduce these discomforts, appropriate sedation is necessary.3 In MAC various sedative drugs are used like benzodiazepines, opioids and propofol and α2 agonist.4 Midazolam is suitable for use with LA for its anxiolysis, sedation, and anterograde amnesia.3 Though it has quick onset can cause prolonged sedation after repeated administration due to longer half-life.5As midazolam has sedative effect with no analgesic property, therefore fentanyl was added as an analgesic. However, no drug is completely complication free. Combination of midazolam with fentanyl increases the risk for hypoxemia and apnea6, 7 while cardio-respiratory depression is seen with propofol.7 Dexmedetomidine, is a selective alpha-2adreno-receptor agonist produces anxiolysis, amnesia, sedation, potentiation of opioid analgesia, and sympatholysis.8 We decided to compare the efficacy of dexmedetomidine and midazolam with fentanyl in tympanoplasty done under LA with the aims to compare the changes in hemodynamic, respiratory parameters and need for rescue sedation/analgesia intra operatively as primary outcome and surgeon satisfaction score as secondary outcomes.
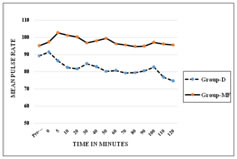
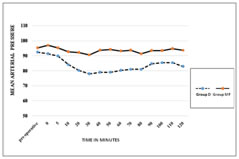
METHODS After approval by the institutional ethical committee this prospective, randomised, double blind, comparative study was conducted in 50 patients aged between 18 to 60 years of either sex with ASA grade І-Ш, posted for tympanoplasty under LA and sedation. Patient refusal for LA, impaired mental status, hypersensitivity to LA or any of the study drugs, coagulation disorders, history of cardiac arrhythmias, sleep apnea, patient’s on treatment with alpha and beta blockers as antihypertensive agent, were excluded from the study. To eliminate the bias, anesthesiologists involve in the perioperative care and patients were all blinded to group assignment. Pre-anaesthetic checkup including routine investigations were done in all the patients, they were explained about the operative procedure and visual analogue scale (VAS) (0-10, where 0 indicated no pain while 10 indicates maximum pain), were explained to the patient. Written informed consent was obtained during pre-operative visit. All the patients kept nil by mouth (NBM) for 6 hours. Non-invasive blood pressure monitor (NIBP), pulse oximeter and Electrocardiogram (ECG) were attached. Baseline pulse rate, systolic and diastolic blood pressure, oxygen saturation(spo2) were observed and recorded. Intravenous(IV) line was secured with 20 gauge IV cannula in opposite forearm and injection Ringer’s lactate(RL) started at the rate of 2 ml/kg. Premedication given with glycopyrrolate(0.004 mg/kg), ondansatron(0.08 mg/kg) ranitidine (1mg/kg) IV. Oxygen was administered throughout procedure through nasal prongs @ 2 L/min. Patients were randomly divided into two groups, randomization was done with computer generated number and kept in a sealed envelope. Group D (n = 25) (control group) patient received IV dexmedetomidine 1 µg/ kg in 30 ml NS over 10 min via infusion pump followed by a continuous infusion of Dexmedetomidine@ rate of 0.2 µg/kg/h (dilution of 1μg / ml). Group MF (n =25) (study group) patient received IV midazolam 0.05 mg / kg plus fentanyl 1.5µg/ kg in 30 ml NS over 10 min via infusion pump followed by continuous infusion of normal saline @ rate of 0.2 ml/kg/h. Sedation was assessed using Ramsay sedation score (RSS) at 5 minute interval. End point was considered as RSS=3 (1=agitated, restless; 2=cooperative, tranquil; 3 = responds to verbal command while sleeping; 4 = brisk response to glabellar tap or loud voice while sleeping; 5 = sluggish response to glabellar tap or loud voice; 6 = no response to glabellar tap or loud voice). If the end point had reached before completing the loading infusion, then the infusion was stopped and required volume noted. After the loading drug infusion if any patient in either of the groups had lesser sedation (a score <3) then IV midazolam0.01mg/ kg was administered which was repeated till RSS 3 was achieved. The maintenance infusion was started immediately in both the groups, once the loading infusions were stopped. ENT surgeon administered LA by infiltrating 2% lignocaine with adrenaline (6-7ml) (1:200000) in the post auricular area. Intraoperative vital data were recorded every 3 min during loading infusion and then every 10-min intervals till the end of surgery. Pain intensity was evaluated using VAS every 5 min initially and thereafter every 10 minutes till it achieved 3. Inadequate analgesia was treated with infiltration of 2% lignocaine with adrenaline (2-3 ml) at the surgical site and noted. If the pain still persist and VAS >3, then rescue IV fentanyl in the dose of 1µg/ kg was given. Total number of rescue doses of fentanyl during surgery was recorded, up to maximum 3 rescue doses each of midazolam and fentanyl were allowed. At any time, if the rescue drug requirement was more than 3, the study drug was discontinued and general anaesthesia was administered and that patient was excluded from the study. The infusions will be discontinued 10- 15 min before end of surgery. Adverse events like hypotension or hypertension (drop or increase in systolic blood pressure or MAP 20% from the baseline), bradypnea (RR <8 breaths/min), bradycardia (HR <50 bpm), desaturation (SpO2<90%), nausea, vomiting, dry mouth or any other event during the procedure was noted. Once the surgery was completed, patients were shifted to the post-operative recovery room and were monitored for vital parameters, degree of analgesia and for adverse events, for 2h. RSS was assessed immediately on arrival in the post-operative recovery room and then every 30 min till patient transfer to surgical ward. Pain was assessed every half hourly for 2 hour, hourly for 8 hour, and then every 4 hourly for 24 hours. The rescue analgesic dose was given at VAS >3 in the form of inj diclofenac 75 mg intra muscularly and was documented. Duration of analgesia was defined as time between the end of surgery till patient required first rescue analgesic. The surgical conditions and satisfaction with sedation technique were graded by surgeon using numerical rating scale (NRS 0-10) with 0 being least satisfied and 10 being most satisfied. Hemodynamics, and need for intraoperative rescue sedation/analgesia were assessed as primary outcome. Sample size calculation was done on the basis of pilot study difference in mean heart rate at five minutes between two groups were mean ± SD (90 ±16.4,106 ±20.4). A sample size calculated by using the formula (2(Zα+Zβ)²*(SD)²/(d)²), power of study was 90% and alpha error was 5% d= difference of mean. According to this formula 22.06 patients per group were required. The sample size of 25 patients were taken in each groups considering the dropouts. All the observations were recorded as mean and standard deviation. All the results were analyzed statistically with MS excel, using students unpaired t test and Chi square test. Statistical significance was considered as significant and highly significant respectively as per P value <0.05 and<0.001. RESULTS In this study the demographic data (age, sex, height, weight, and ASA grading) and duration of surgery (SD± Mean) were comparable in both groups P> 0.05 (Table 1). The baseline mean pulse rate and MAP were comparable in both the groups but were significant after 10minutes from starting of loading dose till post operatively up to 1 hr in group D as compared to group MF (P = 0.001) as shown in figure 1 and 2. Intraoperatively respiratory rates were comparable in both groups. Requirement of rescue sedation, rescue analgesia and local infiltration were lower in group D as compared to Group MF which were significant (Table 2). Duration of analgesia (in minutes) was longer in group D (87.6±16.8) as compared to group MF (19.2±18.7) P>0.001, Surgeon satisfaction score was also statistically highly significantP<0.001 between group D (9 ± 0.27) and group MF (7 ± 0.63). No complications were found in either dexmedetomidine or midazolam with fentanyl group. Not a single patient were excluded from the study.
Table 1: Demographic Data
Table 2: Rescue Sedation, Infiltration and Analgesia
All Values expressed as number, (%) percentage, ** statistically highly significant.
Figure 1: Intra Oprative Mean Pulse Rate Figure 2: Intra Operative Mean Arterial Pressure DISCUSSION For MAC, an ideal sedative agent should have rapid onset, easy titration, high clearance, and minimal side-effects; particularly a lack of cardiovascular and respiratory depression. Due to unavailability of such an ideal sedative agent, techniques for MAC often utilizes a combination of agents to provide analgesia, amnesia, and hypnosis with complete and rapid recovery that suits a particular operative procedure with minimum side effects. Dexmedetomidine has an arousable sedation with analgesic effect, preservation of better airway reflexes, and ventilatory drive.9 As midazolam has sedative effect with no analgesic property, injection fentanyl was added as an analgesic and this combination is conventionally used for MAC.10,11 In this prospective study, we compared the safety and efficacy of dexmedetomidine with midazolam plus fentanyl in MAC for tympanoplasty done under LA. Dexmedetomidine with a loading dose of 1 μg/kg over 10 min was chosen based on previous studies and review of literatures, that on administration of low or moderate doses and slow rates of infusion of dexmedetomidine, α2 agonist effects are observed but not α1 effect.12,13,14 Since dexmedetomidine has a short distribution half-life of 5 min, maintenance infusion is necessary. The maintenance dose is 0.2- 0.8 μg/kg/h. We chose to use an infusion dose of 0.2 μg/kg/h of dexmedetomidine. And for MF group after conducting pilot study, midazolam 0.05mg/kg and fentanyl 1.5 μg/kg as loading dose over 10 min was chosen. Mean Pulse rate and MAP was significantly lower in Group D from 5 and 10 min respectively after starting of the loading dose and remained lower throughout surgery till 1 hour post operatively as compared to Group MF. This reduction might be due to markedly decreased sympathetic activity (central sympatholytic action) that can attenuate the stress response to surgery, reducing tachycardia and hypertension15 and decreases MAP when administered in low or moderate doses and slow rates of infusion.16Similar results were observed in the studies done by other authors17,18,2 Other studies also found that dexmedetomidine has a clinical advantage over midazolam in providing a better operative field (controlled hypotension) for microscopic surgery.4,19 The effect of dexmedetomidine on reducing haemodynamics could be beneficial for patients at risk for cardiac morbidity.20,21 Respiratory rate and SpO2 were comparable in both the groups. Other study found similar findings.17 Dexmedetomidine does not cause respiratory depression because its effects are not mediated by the Ỳ amino butyric system22 while midazolam-opioid combination displays synergism not only in providing hypnosis but also to produce severe respiratory as well as cardiac depression.9 All patients in group D attained an RSS= 3 at the end of loading dose but in group MF 2 patients required a rescue sedation dose after completion of loading dose at 10th min to attain RSS=3, while in other study17 in which none of the patients in both the groups required rescue sedation after completion of loading dose, instead two patients each in both the groups required stopping the loading dose infusion at 8th minute as RSS= 3. In our study 2 patients in group D and 14 patients in group MF required rescue sedation. Immediately on arrival to recovery room, 1 patient in group D and 4 patients in group MF were having an RSS=3 rest had an RSS=2, this probably depended on the time of last rescue sedative and analgesic dose. Requirement of rescue local infiltration was low in Group D (8/25 patient) compared to Group MF (20/25 patient). Only 2 patients in group D required rescue fentanyl as analgesic, while 14 patients in group MF required rescue fentanyl. VAS remained low in group D intra-operatively and post operatively till 90 min as compared to group MF. Similar results were found in other studies.18,23. Dexmedetomidine has an analgesic-sparing effect, and significantly reducing opioid requirements both during and after surgery15and this could be reason for decreased rescue analgesic requirement. Post-operative analgesia was better in group D as compared to group MF. Duration of analgesia (in minutes) was significantly longer in group D (87.6±16.8) as compared to group MF (19.2±18.7) P>0.001, while it was comparable in other study.17 This maybe because of the elimination half life of about 2 h and drug infusion was continued up to the end of surgery. In middle ear surgeries surgeon satisfaction score mainly depends on bloodless surgical field, and less intraoperative patient movement. Surgeons were asked about their satisfaction regarding the sedation technique after completion of surgery. Surgeon satisfaction score was higher in group D than in group MF. Surgeon were highly satisfied with the dexmedetomidine sedation as it had bloodless surgical field19, intra operative less patient movement in group D as compared to group MF. Surgeon satisfaction score was statistically significant between group D and group MF P = 0.001, Similar results were also seen by other studies.2, 23 The limitations of our study is that we have not included patients of higher ASA grade (IV and V) and patients with Pulmonary and CVS comorbidities. Sedation assessment was subjective, which needed clinician – patient interaction and this may lead to awakening of the patients or interrupting the state of sedation they achieved24, 25 instead BIS could have been used. Also interaction with the patient may interfere with the surgeons work especially in microscopic surgeries. We have not assessed the cognitive function and amnesia score as tympanoplasty is not a day care procedure at our institute. The scope of our study is dexmedetomidine can be used in patients with cardiac comorbidities, as the effects of α2 agonists on the cardiovascular system may be beneficial in high-risk patients.26, 27
CONCLUSION Compared to Midazolam plus Fentanyl sedation in Monitored Anaesthesia Care for tympanoplasty done under local anaesthesia, Dexmedetomidine is associated with better haemodynamic stability, with no respiratory depression, lower pain scores and greater surgeon, satisfaction without any adverse effects. Therefore, dexmedetomidine seems to be a better alternative to the combination of midazolam plus fentanyl sedation. However, appropriate patient selection, adequate preparation, and careful monitoring is mandatory.
REFERENCES
|
|
 Home
Home