Official Journals By StatPerson Publication
|
Table of Content - Volume 10 Issue 3 -June2019
The efficacy of two different doses of gabapentin (300mg and 600mg) along with NSAID (diclofenac transdermal patch 100mg) in postoperative pain management following abdominal hysterectomy
Suresh Rajkumar1, V Hari Babu2*
1,2Associate Professor, Department of Anaesthesiology, Aarupadaiveedu Medical College, Kirumampakkam, Puducherry INDIA. Email: sureshrajkumar.manickam@avmc.edu.in, bommidisrk@gmail.com
Abstract Context: Gabapentin, an antiepileptic drug has a role in acute postoperative pain management due to its anti hyperalgesic property. Pre-emptive analgesia is the recent concept in multi modal pain management. The purpose was to analyse the effect of Gabapentin as Pre-emptive analgesic. Aim: To study the efficacy and safety of Gabapentin on pain following abdominal hysterectomy and on reduction in Tramadol consumption. Methods and Materials: A randomized, placebo-controlled, double -blind study conducted on 120 ASA I and II patients planned for abdominal hysterectomy. The patients were randomized to receive either oral placebo or Gabapentin 300 mg or Gabapentin 600 mg 1 hour before surgery along with diclofenac transdermal patch 100 mg. Spinal anesthesia was instituted with injection Bupivacaine 0.5% heavy 15mg. Heart Rate, SpO2, Blood pressure, respiratory rate, pain intensity score, rescue analgesic consumption and patient satisfaction score and side effects were recorded. Statistical analysis: Parameters were analysed using one-way ANOVA, Post Hoc LSD test and Chi-Square test. P-value ≤0.05 was considered statistically significant. Results: The pain intensity scores and total Tramadol consumption were significantly reduced in Gabapentin 600 mg group when compared to Gabapentin 300 mg and placebo. The patient satisfaction score was significantly high in Gabapentin 600 mg. Conclusion: Pre-emptive use of Gabapentin (600mg) combined with Diclofenac transdermal patch (100mg) significantly decreases postoperative pain and rescue analgesic requirement, thus provides an effective means of giving postoperative analgesia following abdominal hysterectomy. Key Word: Pre-emptive analgesia, Gabapentin, abdominal hysterectomy
INTRODUCTION The current concept of multimodal postoperative analgesia is mainly based on the combination of opioids, nonsteroidal anti-inflammatory drugs (NSAIDS) or paracetamol, and perioperative administration of local anesthetic drugs. The use of opioids may be limited by adverse effects such as nausea, vomiting, excessive sedation, pruritis and urinary retention. Postoperative pain affects recovery from surgery and anesthesia1,2. Combination of opioids and non-opioid analgesics improve the quality of postoperative analgesia, reduce opioid requirement and associated side effects. The mechanistic approach to pain management, based on current understandings of the peripheral and central mechanisms involved in nociceptive transmission, provide newer options for clinicians to manage pain effectively. Nowadays, most of the institutions delivering multimodal pain management services using the concept of pre-emptive analgesia, (an analgesic treatment initiated before as opposed to after the surgical procedure) to protect the central nervous system from the deleterious effects of noxious stimuli and the patient from the resulting hyperalgesia, allodynia and increased pain. Gabapentin, has a selective effect on the nociceptive process involving central neuronal sensitizations by reducing the hyper excitability of dorsal horn neurons induced by tissue damage. It binds to the α2δ subunit of the voltage dependent calcium channel, which may explain its anti hyperalgesic action3,4. Recently, several studies shown that Gabapentin may have a place in the treatment of postoperative pain. This drug is relatively well tolerated and belongs to a class of drugs that have anxiolytic properties. This prompted us to explore the pre-emptive analgesic effects of Gabapentin with combination of other analgesic drugs for postoperative pain management.
AIM AND OBJECTIVES The aim of the study was to evaluate the efficacy of two different doses of Gabapentin (300mg and 600mg) along with Diclofenac transdermal patch (NuPatch 100) 100 mg in postoperative pain relief. And also, to assess the effect of Gabapentin on postoperative rescue analgesic (Tramadol) consumption.
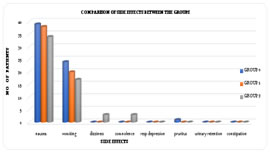
MATERIALS AND METHODS This randomized, placebo-controlled, double blind study was conducted at our tertiary institute on 120 healthy adult females of ASA grade I and II who underwent total abdominal hysterectomy with or without bilateral salphingo oopherectomy under subarachnoid block between 2007 and 2008. Following approval of protocol by the review committee of our institution and informed consent from all patients, they were subjected to routine preanesthetic evaluation to rule out any anatomical and systemic disorders. Cooperative patients with minimum age of 18 years, who were over 40kg and had no psychologic problem could participate in this study. Exclusion criteria included body weight exceeding 20% of ideal body weight, age older than 65 years or younger than 18 years, allergy to drugs, concomitant medical diseases (hypertension, bronchial asthma, diabetes mellitus, bleeding disorders, decreased platelet count, hepatic and renal disease), local or systemic sepsis, history of drug abuse (alcohol/narcotic drugs), significant vertebral column deformity, patients’ refusal and use of opioid analgesics or Gabapentin within 24 hours before surgery. If surgery time exceeds 2 hours were also excluded. During preoperative visit patients were educated about study plan, VAS andVRS scoring and the way of postoperative pain control. On the assumption that Gabapentin low dose (300mg) and high dose (600mg) decreases the postoperative Tramadol consumption by 24% and 29% respectively, according to power analysis, this study requires 40 patients in each group for 80% power and 95% confidence limits with a significance of P <0.05. All the 120 cases were assigned into three groups by simple randomization using sequentially numbered, opaque sealed envelopes. Group 0 (n=40) received two placebo capsules, Group 1(n=40) received one placebo capsule and one Gabapentin (300 mg) capsule and Group 2 (n=40) received Gabapentin (600 mg) as two capsules orally 1 hour before surgery preoperatively. At the same time a Diclofenac transdermal patch (NuPatch 100) (100 mg) was applied over the arm. The same was repeated after 24 hours postoperatively. The study medication was given as 2 capsules orally with a sip of water one hour before surgery. The study drug administration and subsequent assessment was done by the same anesthesiologist, who was blinded to group allocation. No other premedication was given at this time. On arrival to the operation theatre, they were preloaded with 10 ml/kg Lactated Ringers solution. Spinal anesthesia was instituted with Bupivacaine 0.5% heavy 15mg into the subarachnoid space at L3-L4 level by 25G Quincke type spinal needle under aseptic precautions. If the height of sensory block reached T6, the surgeon was allowed to start surgery. Operation was performed via Pfannenstiel incision. All the vital parameters (Heart Rate. Blood Pressure, ECG, and SpO2) were monitored intraoperatively. Fluid administration was continued intraoperatively and hypotension, if any was treated with fluid replacement and vasopressors. Pain intensity was assessed postoperatively by Visual Analogue Scale (VAS) and Verbal Rating Scale (VRS 0-100). Postoperative pain assessment was done according to 100 mm VAS scale where 0= no pain.100= the worst pain. Any patient with VAS ≥30mm or VRS≥50 were administered injection Tramadol 1 mg/kg diluted with 20ml Normal Saline intravenously over 10 minutes as rescue analgesic. Time to first dose of rescue analgesic received was recorded. The total no of doses of rescue analgesic received by the patient at 24 hours, 48 hours were recorded. Both VAS andVRS were assessed at rest, on coughing and on movement. Both VAS and VRS were observed at 1, 2, 4, 6, 12, 24, 36 and 48hour. Patient satisfaction with postoperative pain management was assessed using a 100-point VRS, with 1=highly dissatisfied to 100= completely satisfied. Patient satisfaction score was recorded at 24 and 48 hours. Ramsay sedation scale5, was used to assess postoperative sedation. Occurrence of postoperative nausea and vomiting and need for Antiemetic therapy was observed. If patient experiences sustained nausea (lasting longer than 5 minutes) or vomiting, injection Ondansetron 4mg bolus dose was given intravenously. Total dose of Ondansetron administered was recorded. Occurrence of other side effects like dizziness, somnolence, respiratory depression (RR< 8 breaths per minute), bradycardia, hypotension, pruritus, urinary retention and constipation were also recorded. Early return of bowel sounds was observed to assess the early recovery of the patient. No other analgesia was administered. The statistical analysis was performed using SPSS software (version 10.0). Demographic data, hemodynamic data, pain scores (VAS andVRS), patient satisfaction score, total Tramadol consumption and total anti emetics received for all the groups at different time points were analyzed using one-way ANOVA. For multiple comparisons between the groups, Post hoc LSD test was used. The Chi-square test was applied to analyses sedation scores and side effects. Data was reported as mean value ± S.D (standard deviation) and numbers (n). P value ≤ 0.05 was considered statistically significant. RESULTS All 120 patients enrolled in the study were completed the study protocol and included in the data analysis. Demographic data including age, body weight, height and ASA physical status and preoperative vitals like pulse rate, systolic and diastolic blood pressure were comparable between all the groups. [table 1] The postoperative pulse rate measured at 4th hour and 6th hour was significantly lower in Gabapentin 600 mg group. Both VAS and VRS at Rest measured at 2nd hour and 4th hour was significantly less in Gabapentin 600mg group. Both VAS and VRS on Cough and Movement measured at 4th hour and the VRS on Cough at 2nd hour was significantly less in Gabapentin 600mg group. [table 2] The time interval to receive first dose of rescue analgesic was significantly delayed in Gabapentin 600mg group. The time interval to receive first dose of rescue analgesic was not significant between placebo and Gabapentin 300mg. In our study the total Tramadol consumption was significantly reduced in Gabapentin 600mg group (291.5±46.32mg; P<0.05) compared to placebo (393.75±57.73mg) and Gabapentin 300 mg (371.25±75.55). The total reduction in Tramadol consumption in Gabapentin 600mg group was 17% in 24 hours, 26% in 48 hours. However, there was no significant reduction in Tramadol consumption between Gabapentin 300 mg and placebo. The total number of doses of rescue analgesic received in 24 and 48 hours in Gabapentin 600mg group was statistically most significant, but not significant between placebo and Gabapentin 600mg. The total dose of rescue analgesic received in 24 and 48 hours was significantly reduced in Gabapentin 600mg. [table 3] The patient satisfaction score at 24 and 48 hours were significantly high in Gabapentin 600mg. There was no significant difference found regarding the sedation score between the study groups. [table 4] There was no significant difference found in incidence of side effects between the groups. But the incidence of nausea and vomiting were less in Gabapentin 600mg (34 and 17) than placebo (39 and 24) and Gabapentin 300 mg (38 and 20). The total number of antiemetic (Ondansetron) doses and the total dose of antiemetic (Ondansetron) received in 48 hours was significantly less in Gabapentin 600mg, but not significant between placebo and Gabapentin 300 mg. Side effects included Dizziness and somnolence found in three patients (Gabapentin 600mg) and Pruritis found in one patient (placebo). Return of bowel sounds in the early postoperative period was not significant. Table 1: Demographic characteristics, preoperative vitals – comparison
(P value ≤ 0.005 is significant) values presented as Mean±SD or (no of patients) 1
Table 2: Comparison of Postoperative pain scores (VAS) at various time interval
(*P value ≤0.05significant) Values presented as Mean±SD Table 3: comparison of rescue analgesia (tramadol) received over 48 hours
(*P value ≤0.05 is significant) Values presented asMean±SD, in hr1, in no of doses2, in mg3. Table 4: Comparison of patient satisfaction scores (PSS) - Verbal Rating Scale (VRS 1-100)
(*P value ≤ 0.05 is significant) Values presented as Mean±SD
Figure 1: Comparison of side effects between the groups
DISCUSSION Multimodal (or "balanced") analgesia involves the use of more than one modality of pain control to obtain additive (or even synergistic) beneficial analgesic effects while reducing opioid-related side effects6. There is now considerable interest in the use of Gabapentin for postoperative pain relief. Animal experiments suggest that the antinociceptive effects of Gabapentin correlated with the suppression of noxious-evoked release of excitatory amino acids in the spinal cord. The majority of researches evaluating Gabapentin as an adjuvant have used 300mg – 1200mg as a single dose in various surgical procedures. In our study we compared the efficacy of two different doses of Gabapentin (300mg and 600mg) along with NSAID (Diclofenac transdermal patch 100 mg) for postoperative analgesia in abdominal hysterectomy cases. Turan et al (2004), studied Gabapentin 1200mg in post-operative pain relief in abdominal hysterectomy as well as in lumbar discectomy in two different studies7. They found 36% (Gabapentin vs placebo) reduction in opioid consumption in abdominal hysterectomy vs 62% (Gabapentin vs placebo) in lumbar discectomy. This difference in rescue analgesic consumption was well explained by Mathiesen (2007), in his research article on Gabapentin on postoperative pain8. He explained that pre and per operative nerve damage in a higher degree contributes to post-operative pain in spinal surgery than in abdominal hysterectomy. Gabapentin is effective in the treatment of chronic neuropathic pain, and would result in a larger analgesic effect in postoperative pain after spinal surgery than abdominal hysterectomy. Turan et al (2004) found 36% reduction in total Tramadol consumption in 24 hours. Gabapentin dose was high in their study (1200mg) compared to our study7. However, when we look at 48 hours reduction in analgesic consumption in our study (26%), it was nearly similar to the reduction in analgesic consumption seen by Turan et al (2006) in 72 hours9. Pandey et al (2005) investigated the effect of different preoperative Gabapentin doses (300-600-900-1200 mg) in single level lumbar disc surgery and found that the dose over 600mg did not decrease Fentanyl consumption i.e. no additional analgesic effect over 600mg10. In our study the sedation scores were statistically not significant (P>0.05) even though the large numbers of patients with higher scores were belonging to Gabapentin 600 mg group at all the measured time intervals. This correlates with the findings of Turan et al (2004 and 2006)7,9. Gilron et al found significant sedation in Gabapentin group which may be due to the high dose (1800mg) of Gabapentin used in his study11.
CONCLUSION Gabapentin 600mg effectively reduces pain intensity in immediate postoperative period and significantly delays the time to first dose of rescue analgesic and significantly reduces the postoperative rescue analgesic requirement along with higher patient satisfaction. Gabapentin 600mg significantly reduces the anti-emetic requirement with decreased incidence of nausea and vomiting with less side effects. To conclude, our study proves that Gabapentin 600mg combined with Diclofenac transdermal patch (100mg) is safe and provides an effective means of giving postoperative analgesia in abdominal hysterectomy patients.
REFERENCES
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home