Official Journals By StatPerson Publication
|
Table of Content - Volume 11 Issue 1 -July 2019
Vishalakshi Patil1, Lohit Kondikar2*, Balaraju T C3, Chaitanya Ghanta4, A. Sanjana Reddy5
1Associate professor, 2Assistant professor, 3Professor and HOD, 4IIIrd year Resident, 5IInd year Resident, Navodaya Medical College, Hospital and Research centre, Mantralayam road, Navodaya Nagar, Raichur – 584103, Karnataka, INDIA.
Abstract Background: Nalbuphine is a synthetic opioid, with kappa agonist or partial μ antagonist action. when added as adjuvant to intrathecal Bupivacaine acts on kappa receptors in the dorsal horn of the spinal cord producing analgesia. Aim: To evaluate the Onset and duration of sensory blockade and motor blockade, duration of post-operative analgesia, and adverse effects of different doses of Nalbuphine with Bupivacaine for spinal anaesthesia. Materials and Methods: Prospective Randomized controlled study done on 100 patients undergoing lower abdominal and lower limb surgeries under subarachnoid block. We randomly allocated four Groups A, B, C, and D, where Groups B, C, and D to receive 1.0, 1.5, and 2.0 mg Nalbuphine made up to 0.5ml with normal saline added to 3.0ml of 0.5% hyperbaric bupivacaine respectively, and Group A receive 0.5 ml of normal saline added to 3.0ml of 0.5% hyperbaric bupivacaine (3.5ml). Assessment of motor and sensory blockade was done by Bromage scale and pin prick method. Pulse rate, NIBP, Respiratory rate, SpO2, and VAS score were monitored intraoperatively and 24hrs post-operative period. Results: The mean onset time of sensory block of Group C and D was significantly early as compared to the onset of sensory block as compared to Group A and B. Duration of two-segment regression of sensory block, duration of motor blockade, and duration of analgesia time were prolonged in Groups C (1.5mg) and D (2.0mg) and found to be significant. The incidence of adverse effects was frequently higher in Group D (P < 0.05) compared to other groups. Conclusion: Nalbuphine is effective adjuvant in spinal anaesthesia. Addition of 1.5mg of Nalbuphine to 0.5% hyperbaric Bupivacaine for subarachnoid block provides excellent analgesia with longer duration of action compared with 1.0 and 2.0mg without adverse effects. Key Words: Bupivacaine, Nalbuphine, Subarachnoid block.
INTRODUCTION Regional anaesthetic techniques of spinal anaesthesia offer many advantages over general anaesthesia including reduced stress response to surgery with postoperative analgesia. The surgical stress response peaks during the postoperative period and has major effects on almost all body systems. A pain-free and stress-free postoperative period definitely helps in early mobilization and recovery, thereby reducing morbidity and mortality. Most commonly used adjuvants are opioids, alpha-2 adrenergic agonist, ketamine, midazolam, etc., but certain side effects such as pruritis, respiratory depression, nausea, vomiting, and urinary retention were observed with opioids1,2.Nalbuphine is a µ receptor antagonist and ĸ receptor agonist3,4. When Nalbuphine is added as an adjuvant to intrathecal Bupivacaine, it has potential to provide good intraoperative and post-operative analgesia with decreased incidence of μ receptor side effects like respiratory depression5,6.Here we compared the effect of Nalbuphine addition at different dosages i.e., 1.0mg, 1.5mg and 2.0mg to 0.5% hyperbaric Bupivacaine intrathecally with 0.5% hyperbaric Bupivacaine alone for duration, quality of post-operative analgesia and any side effects.
MATERIALS AND METHODS The study was approved by the local institutional ethics committee and written informed consent was obtained from all patients before participation.About 100 patients of American Society of Anaesthesiology (ASA) I and II, aged 18-60 years, both sexes posted for elective lower limb surgeries and lower abdominal surgeries under spinal anaesthesia included in the study. Inclusion Criteria: ASA physical status I and II. Patients of either sex. Patients aged between 18-60years. Patient with written valid consent. Patient undergoing elective lower abdominal and lower limb surgeries. Exclusion Criteria: ASA III and IV grade. Lack of valid informed written consent. Infection at the subarachnoid block injection site. Patients with neurological and musculoskeletal disease. Patients with bleeding disorders. Patients on anticoagulants. Pregnancy. History of allergy to local anaesthetic. Patients on tranquilizers, hypnotics, sedatives, and other psychotropic drugs. Patients were visited and detailed pre-anaesthetic examination done. Preparation of patients includes period of overnight fasting. Pre-medication done with oral tablet alprazolam 0.5mg and tablet Ranitidine 150mg given at night and morning on the day of surgery. The procedure of spinal anaesthesia was explained to the patients and informed written consent was obtained. The patients were educated about the use of visual analogue scale (VAS). Routine investigations like complete hemogram, blood sugar, electrocardiogram, chest X-ray were done. Ampoule containing preservative free Nalbuphine hydrochloride 10mg in 1 mL (NEON LABORATORIES LTD.) was used. The dose of intrathecal Nalbuphine was measured using insulin syringe. In the Operating room, IV access was obtained on the forearm with 18 Gauge IV cannula and IV infusion started with Ringer Lactate or Normal saline. Monitors connected to the patients include three lead ECG in standard lead 2, non-invasive BP and pulse oximeter. Patient’s basal parameters were recorded prior to spinal anaesthesia. Spinal anaesthesia was performed with the patient in the lateral decubitus position or sitting position using a 25-gauge Quincke needle at the L3–L4 or L4–L5 interspaces. Following free flow of CSF, respective drug was injected into subarachnoid space as mentioned in Table 1 and were positioned supine immediately. After the spinal block, intra-operatively and post-operatively vitals were observed and recorded at 5 min, 10 min, 15 min, 30 min, 1 hr, 2 hr, 4 hr, 6 hr, 12 hr, 24 hr. The following Parameters were noted and used for Comparison between the Groups.
Pain was assessed by VAS (visual analogue scale). Here patient was given a scale marked from 0-10 and was asked to mark on the scale the degree of pain he /she experiencing from 0-no pain to 10 maximum pain ,when VAS > 3, rescue analgesia given with inj. Diclofenac sodium and study ended. STATISTICAL ANALYSIS Statistical analysis of all the data was done by using One-way ANOVA, Sample t-test and Wilcoxon test. P value of 0.05 or less is considered significant for statistical analysis.
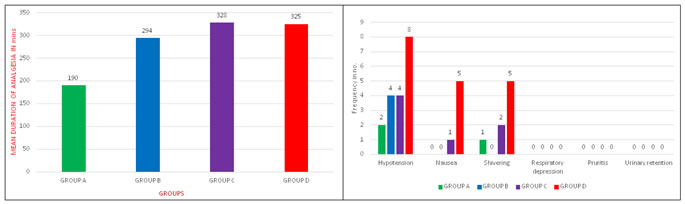
RESULTS Patients were randomly allocated into four groups. Each group consists of 25 patients. They received either of drug solution as below (Table 1).
Table 1: Group Distribution.
The effects of intrathecal 0.5% hyperbaric Bupivacaine with Nalbuphine hydrochloride at three different doses (1.0, 1.5, and 2.0 mg) was studied and compared with 0.5% hyperbaric Bupivacaine alone in 100 patients belonging to ASA grade I and II who underwent lower limb surgeries and lower abdominal surgeries. The four Groups A, B, C and D did not differ significantly in demographic characteristics like Age, Sex, Weight and Height as shown in Table – 2. The results regarding the characteristics of Sensory block and Motor Block, Duration of Effective Analgesia were summarized in Table – 3.The mean time of onset of Sensory block and Motor block between the Groups are comparable with P – value <0.05 which are statistically significant (Table – 3). Two-segment regression time of Sensory blockade was progressively prolonged in Groups B, C, and D compared to Group A (Table – 3). Group D recorded with mean of 175.2 min compared with 166.5 min in Group C, in Group B 129.4 min and Group A 121 min. The duration of Motor blockade also prolonged progressively in Groups B, C, and D compared to Group A (Table - 3). Group D recorded with the longest duration of Motor blockade with a mean of 172.2 min compared to Group C 163.0 min, Group B 129.84 min, and Group A 117.0 min (Table - 3). The duration of Analgesia was prolonged progressively in Group B, C, D compared to Group A (Table – 3 and Figure – 1). Group C recorded with the longest duration of Analgesia with a mean of 328.2 min compared to Group D 325.2 min, Group B 267.9 min, and Group A 154.8 min (Table – 3 and Figure – 1).The side effects of Hypotension, Nausea, Vomiting, Shivering, Pruritis, Respiratory depression and Urinary retention are more common in Group D compared to other groups (Table – 4 and Figure – 2).
Table 2: Demographic Characteristics
Table 3: Summary of Results
Table 4: Side Effects
Figure 1: Duration of Analgesia Figure 2: Incidence of Side Effects DISCUSSION Nalbuphine, a mixed agonist-antagonist drug, binds both to mu and kappa receptors, but its action on these receptors is divergent. When Nalbuphine binds to μ receptor, it serves only to competitively displace other μ agonists from the receptor without itself displaying any agonist activity similar to those of naloxone. However, when it binds to kappa receptor, it has agonist activating effect. This pattern of binding and effects defines Nalbuphine as a mixed agonist-antagonist. Nalbuphine, administered Intrathecally, binds to Kappa receptors in the brain and spinal cord areas which are involved in nociception, producing analgesia and sedation without µ side effects. The rationale for the combination of opioids and local anaesthetics is that these two types of drugs eliminate pain by acting at two different sites. Local anaesthetics act at the nerve axon and the opioid at the receptor site in the spinal cord7,8. The major site of action of opioid is within the second and third laminae of substantia gelatinosa in the dorsal horn of the spinal cord. Intrathecal opioids used as adjuncts are capable of producing analgesia of prolonged duration, but allow early ambulation of patients because of their sympathetic and motor nerve-sparing activities. The popularity of intrathecal opioids was undermined by reports of side-effects such as respiratory depression, pruritus and postoperative nausea and vomiting. There are few studies suggest that neuraxial administration of Nalbuphine has minimal side effects such as respiratory depression, pruritis, nausea, vomiting, and significant prolonged duration of analgesia5. In our study, all the four groups were comparable in distribution of patients according to age, sex, weight, and height. The mean onset time of sensory block and motor block of Group C and D was significantly early as compared to Group A and B. Two segment regression of sensory blockade was significantly prolonged by addition of intrathecal Nalbuphine as seen in Group B, C and D when compared with Group A Bupivacaine alone and this result correlates with that of study done by Jyothi B, Shruthi Gowda, Safiya l Shaikh who had compared 100 patients undergoing lower abdominal and lower limb orthopaedic surgeries under subarachnoid block. They used different doses of Nalbuphine 0.8mg, 1.6mg, 2.4mg added to 0.5% heavy Bupivacaine and they concluded that addition of 0.8mg of Nalbuphine to 0.5% Bupivacaine for subarachnoid block provides excellent analgesia with longer duration of action compared with 1.6 and 2.4mg of Nalbuphine9. Tiwari et al., who compared intrathecal Nalbuphine 0.2mg and 0.4mg added to hyperbaric Bupivacaine with Bupivacaine alone. They concluded that prolonged duration of analgesia was seen in Nalbuphine 0.4mg without adverse effects.10 Mukherjee et al. had compared 100 patients undergoing orthopaedic lower limb surgeries under spinal anaesthesia. They used different doses of Nalbuphine 0.2, 0.4, and 0.8 mg added to 0.5% Bupivacaine and they concluded that 0.4 and 0.8 mg have significant prolonged duration of analgesia11. Nalbuphine also provided hemodynamic stability. Similar findings are seen in the study conducted by Culebras et al.6, Tiwari et al.10, Mostafa et al.12, where there was no gross hemodynamic changes throughout their study. From our study, we can conclude that use of Nalbuphine hydrochloride along with Bupivacaine causes no gross hemodynamic disturbances even with increasing the dosage from 1.0mg to 2.0mg. In our study, none of patient had respiratory depression (respiratory rate below 10 bpm, SPO2 <90%). Nalbuphine exhibits ceiling effect for respiratory depression. This is proved in studies done by Romagnoli and Keats13, Thomas et al14. Since respiratory depression is predominantly µ receptor-mediated and Nalbuphine is a µ receptor antagonist, respiratory depression effect is expected to be attenuated by Nalbuphine. Increasing the dosage from 1.0mg to 2.0mg did not cause any respiratory complications. This result correlates that of the studies done by Culebras et al.6, Tiwari et al.10, and Mostafa et al12. Since Nalbuphine is a mixed agonist-antagonist, studies by Pugh and Drummond GB15, Thomas et al.14, have proved that it exhibits a ceiling effect to analgesia that is increasing doses of drug produce increasing analgesia only up to a certain point. Beyond that point, further increase in dose does not result in increased intensity of analgesia. In our study, Nalbuphine exhibits analgesic ceiling effect at 1.5 mg dosage, above which will not increase analgesic efficacy. This analgesic ceiling effect can be a significant limitation of Nalbuphine usage. In our study, addition of Nalbuphine significantly prolonged duration of analgesia which correlates to results of studies done by Lin16, Culebras et al.6, Tiwari et al.10, Mostafa et al12. Patient who received Bupivacaine alone had significantly higher pain scores earlier than patients who received Nalbuphine-Bupivacaine combinations as assessed by VAS. Studies done by Tiwari et al.10, Mostafa et al.12, also reported that Nalbuphine prolonged duration of analgesia with reduced VAS pain score. Adverse effects like nausea, vomiting, urinary retention, and shivering were statistically insignificant in Group B (1.0mg) and Group C (1.5mg) comparing with patients in Group D (2.0mg). Nalbuphine 1.5mg (Group C) has significant prolonged duration of analgesia with minimal adverse effects (P < 0.0001) than Nalbuphine 2.0mg (Group D), while Nalbuphine 1.0mg (Group B) have significant lesser duration of analgesia compared to Group C and D. Nalbuphine 2.0mg (Group D) have prolonged duration of analgesia but increased adverse effects. With all the above observations we conclude that addition of Nalbuphine to Bupivacaine provides prolonged analgesia, and superior pain relief with minimal side effects in Group C (1.5mg) compared to the other Groups B and D and Bupivacaine alone in spinal anaesthesia. Since Nalbuphine reaches ceiling effect at lower intrathecal dosage i.e, at 1.5mg the increased drug dosage is not required and this increases the safety margin.
CONCLUSION We conclude that intrathecal Nalbuphine 1.5mg added to 0.5% hyperbaric Bupivacaine for spinal anaesthesia in patients undergoing lower abdominal surgeries and lower limb surgeries provides excellent analgesia with prolonged duration of sensory block and motor block without adverse effects.
REFERENCES
. |
|
 Home
Home