Official Journals By StatPerson Publication
|
Table of Content - Volume 11 Issue 1 -July 2019
An observational study of Baska mask: A third generation supraglottic airway device
Ashwini B*, Balakrishnan**, Beula G***
*Junior Resident,**Professor,***Assistant Professor, Department of Anaesthesia, Sree Mookambika Institute of Medical Sciences, Kulasekharam, Tamil Nadu, INDIA. Email: beulakiron@gmail.com
Abstract Background: The ideal Supraglottic airway device must have high airway seal pressures during spontaneous and positive pressure ventilation and also low resistance to the flow of gases. Baska mask is a 3rd generation Supraglottic Airway Device (SGA), having a non-inflatable cuff with the better sealing pressure that increases with intermittent positive pressure ventilation (IPPV) without gastric inflation and novel gastric drainage system that reduces the risk of gastric aspiration. Aim: To determine whether the salient features and improvements incorporated in the Baska Mask® provide a clinically useful SGA. Materials and Method: Fifty consecutive patients (American Society of Anesthesiology physical status grade I–III, aged 18–60 years) from a population of elective surgery patients were selected. A variety of surgical interventions (general surgery, orthopedics and Otorhinilaryngology) not requiring endotracheal intubation received a single-use Baska Mask® for anesthesia. The insertion success rates, insertion time and effective airway maintenance was observed. Post-extubation, the patient was assessed for coughing, blood staining, trauma and sump clearance. Postoperative airway morbidity in the form of sore throat, dysphagia, and dysphonia were evaluated at the end of surgery. Informed consent was obtained. Descriptive and analytical statistics were performed by SPSS version 19. A P value of less than 0.05 was considered statistical significant. Results: The airway was secured with Baska mask in all the patients with the first attempt rate of 88% and second attempt rate of 92%. The average insertion time was 16 ± 6 seconds. Conclusion: The new SGA Baska Mask® has many novel features which should improve safety when used in both spontaneously breathing and IPPV anaesthesia. Key Words: Supraglottic Airway Device, Extraglottic Airway Device, Baska Mask
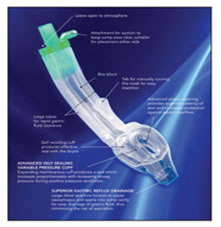
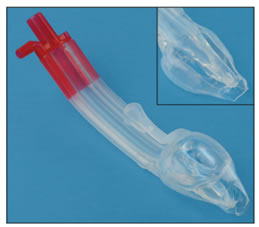
INTRODUCTION The era of development of Supra Glottic Airway (SGA)/EAD devices to secure the airway started back in 19001. Miller proposed a classification based on the fact that EADs are differentiated by virtue of where in the hypopharynx the cuff of the device(whether it be inflatable or anatomically pre‑shaped) provides a seal, whether or not the sealing effect is directional, and whether or not esophageal sealing occurs.2 The Baska Mask® designed by Australian anesthetists Kanag and Meena Baska, is a new CE-‐approved and internationally patented EAD, provided in single use and multi-use versions.4The Baska® mask obviates the need for an orogastric tube and replaces this with a sump and two drains.
Specific features of the Baska Mask® airway5 Cuff
Sump Area
Airway Tube
Shape and Design
AIMS AND OBJECTIVES To determine whether the salient features and improvements incorporated in the Baska Mask® provide a clinically useful SGA
MATERIALS AND METHODS After obtaining Informed consent, fifty consecutive patients (American Society of Anesthesiology physical status grade I–III, aged 18-60 years) patients posted for elective surgery were selected. A variety of surgical interventions (General surgery, Orthopedics and Otorhinolarygology) not requiring endotracheal intubation received a single-use Baska Mask® for anesthesia. Patients were excluded from the trial if they had a known or predicted difficult airway, a mouth opening of <2.5 cm, were at increased risk of aspiration of gastric contents, required surgery in the non-supine position or were undergoing head and neck surgery. The pre-anesthetic evaluation including the patients BMI, Thyromental distance, Mallampati grade, Inter‐incisor distance, their dentition and neck movement were evaluated. After removal from its sterile packet, the integrity and function of the Baska Mask® was checked by occluding the airway opening of the proximal connector end with one thumb, holding the mask head with the other hand and placing the other thumb over the airway opening of the mask to seal. Size selection was based on the manufacturer’s recommendation of weight-based estimate plus clinical judgement (Table 1). The entire body of the mask was lubricated with a water-based lubricating gel. OBSERVATION AND RESULTS
Table 1: Recommended Size
A standard anesthesia sequence protocols was followed. All EADs were inserted in strict accordance with the manufacturer’s recommendations. The patency of the airway was ascertained and the Baska Mask® was connected to the breathing circuit and fixed in place with adhesive tape. A clear airway was defined as SpO2 >95%, ETCO2 <50 mmHg and tidal volumes >6 ml/kg. At the end of surgery, the anesthetic gas mixture was replaced with 100% oxygen to facilitate patient recovery. The Baska Mask® was removed when protective reflexes returned to normal. The following assessments were made: (1) The success of insertion was assessed by the number of insertion attempts (counted as an attempt when the EAD is taken in and out of the mouth); (2) the ease of insertion (very easy, easy, difficult, very difficult); (3) the insertion time (the time between picking‐up the prepared mask and successful placement);(4) the effective airway time (time between picking‐up the prepared mask and obtaining the first capnograph trace);(5) The anatomical position of the Baska Mask® in situ was assessed clinically (by observing that the midline of the device remained in the anatomical midline); (6)ease of removal of the device (very easy, easy, difficult, very difficult); (7) whether following extubation, the patient was (a) coughing, there was (b) blood staining on the device, or(c) trauma to lips, tongue, and teeth, (d) whether the sump clearance was adequate or not (i.e. whether the sump was clear of mucous and gastric contents, and (e) whether or not there was gastric fluid in the airway cavity; and (8) postoperative airway morbidity sore throat, dysphagia, dysphonia graded as none, mild, moderate, or severe, at 2 h in the post operative recovery room.Intra-operative complications were recorded as well as any intervention required to correct the use of the EAD. At the time of removal of the mask, its shape and integrity were checked thoroughly.
Table 2: Patient’ characteristics (N = 50)
Table 3: Performance data (N = 50)
Out of the 50 participants Male to female ratio was 1:2.3 and most of them belonged to age group of more than 40. The first attempt to successfully insert a Baska Mask® was high(88% of the patients). Five patients (10%) needed a second attempt and one patient (2%) a third attempt. Insertion was considered very easy in 36 patients (72%), easy in 13 patients(26%), and difficult in 1 patient (2%). Insertion time and effective airway time were respectively 16±6 and 32 ±12 seconds. In two patients, a smaller size Baska Mask® was used (size 4 instead of size 5) because of leakage and difficulties obtaining a normal, sustained capnogram trace waveform. The results from the second mask insertion were incorporated in the study. Clinical assessment of the “device midline” corresponded to the “anatomical midline” position in all patients. The majority of the patients, i.e. 36 (72%) breathed spontaneously, while the others were temporarily ventilated mechanically. The mean maximum CO2 measured was 5.7±0.7 volume %, and the mean duration of anesthesia was 58±32 min. At the end of surgery, the Baska Mask® could be removed easily without problem in all patients. None of the masks lost their integrity or shape. Coughing during extubation was seen in two patients, blood staining on the device in four patients, and trauma to the lip in one patient. Airway morbidity was measured in the recovery room two hours postoperatively, revealed minor sore throat in five patients and dysphagia in one patient. The list of intra‐operative complications was small and insignificant. DISCUSSION Most EADs which depend upon an inflated cuff to maintain a seal, pose potential hazards as the cuff pressure may be either too high or too low6. Over-inflation may cause airway trauma, while under‐ inflation may result in airway leak and potential aspiration of gastric content. With the Baska Mask®, neither cuff deflation prior to insertion nor cuff inflation, nor the use of a cuff pressure measurement device nor monitoring of cuff pressure during the surgical intervention is required7. The cuff of the Baska Mask® does not rely on inflation of a cushion or balloon to retain its shape or maintain a seal during use.8,9 The soft diaphragm Bask mask cuff distends during the inspiratory phase of positive pressure ventilation increasing the effectiveness of the seal. The severity of throat discomfort, dysphagia and dysphonia is very low. Removal of device was also considered easy. The whole length of the airway tube incorporates a bite block and two “clearance” gastric drain tubes, all features of a third-generation EAD. Performance data suggests that it is feasible to obtain adequate or good outcomes in the overall majority of adult patients, with a high first attempt success rate(88%), easy insertion rate (92%), short insertion time (16 s),and fast effective airway time (32 s).No patient had to be converted to another EAD. An advantage of the dual gastric drain tube is that one drain tube can be used to apply low pressure suction all the times. The other gastric drain tube serves as (1) a free air vent and (2) a guide for an OGT, which can be used to empty the stomach. Aspiration of gastric content through the OGT verifies the correct position of the gastric tube.13
CONCLUSION The new EAD Baska Mask® has many novel features which should improve safety when used in both spontaneously breathing and IPPV anesthesia. It is another step forward in the search for an ideal extraglottic airway device, which incorporates an airway tube, a tab to help negotiate the palato-pharyngeal curve, two large tubes entering the sump area for high suction clearance of the sump, a large sump reservoir to collect any fluid entering the pharynx, a bite block over the full length of the airway tube, an oval, anatomically‐ curved airway tube and a non-inflatable silicone mask (which adjusts to the contours of the mouth and pharynx, bringing the aperture of the mask towards the glottic entrance).
REFERENCES
. |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home