Official Journals By StatPerson Publication
|
Table of Content - Volume 11 Issue 2 -August 2019
Peripheral opioid receptor mediated enhancement of analgesia by buprenorphine in intravenous regional anaesthesia
Nikhil Swarnkar1*, Anshul Yadav2
1Assistant Professor, 2Resident, Department of Anaesthesiology and Intensive care, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Sawangi, Wardha, Maharashtra, INDIA.
Abstract Background: Recent demonstration of opioid receptors along peripheral sensory nerves especially after painful inflammatory conditions forms the basis of peripheral opioid analgesia. This form of opioid antinociception has the potential to help overcome one of the disadvantages of intravenous regional anesthesia (IVRA) that is lack of postoperative analgesia. The aim of the study was to assess the efficacy of Buprenorphine as an adjunct to Lignocaine in (IVRA) for postoperative analgesia. Methods: Seventy-five consenting patients undergoing hand and forearm surgery were randomly allocated into three groups of twenty-five each: group A received 0.5% 40 ml Lignocaine for IVRA, group B received 0.5% 40 ml Lignocaine for IVRA and Buprenorphine 0.3 mg intramuscularly and group C received 0.5% 40 ml Lignocaine with Buprenorphine 0.3 mg for IVRA. Postoperative analgesia was assessed using visual analog scale (VAS) on a 0 to 100 mm scale in the immediate postoperative period and 1 hourly thereafter for 24 hours. Patients were given Declofenac 1 mg/kg orally whenever VAS score exceeded 25 or patient demanded analgesic. Results: Onset time for sensory block was longer in group C as compared to group A and B (5.0±1.0 min versus 4.0±0.6 and 4.0±0.4) whereas motor block onset time was comparable in all the three groups. Quality of sensory and motor block was similar in all the groups. Duration of postoperative analgesia was significantly longer in group C (1200 ±120 min.) as compared to 42±12 and 420±36 minutes for group A and B respectively (p=0.001). Analgesic consumption was also significantly lower in group C (56±9 mg versus 201±27 and 120±24 mg for group A and B respectively (p=0.001). Incidence of nausea/vomiting and sedation was much higher in group B as compared to other groups (p=0.002).Conclusion: We concluded that addition of Buprenorphine 0.3 mg to Lignocaine for IVRA significantly prolongs analgesia without causing systemic side effects. Key Words: Peripheral opioid receptor.
INTRODUCTION
METHODS The prospective randomized double-blind study was carried out between 2009-10 at Datta Meghe Institute of Medical Sciences, Wardha, India on 75 patients scheduled for forearm or hand surgeries like open reduction and internal fixation of both bone forearm, tendon repair and K-wire fixation. The approval of institutional ethical committee on research and informed consent from patients was obtained. Thorough history, clinical examination and routine investigations including any special investigation, if required were carried out. Patients of either sex, between age group of 18 to 60 years and ASA I and II were included in the study. Patients with known hypersensitivity to local anesthetics, peripheral vascular disease, where use of tourniquet was not possible/contraindicated, sickle cell anemia, diabetes mellitus, cardiovascular diseases like myocardial infarctioncardiac arrhythmias were not included in the study. Patients were allocated randomly into three groups: Group A (control group 1, n=25) - received 40 ml, 0.5% preservative-free Lignocaine (Xylocard®, Astrazeneca, India)Group B (control group 2, n=25) - received 40 ml, 0.5% Lignocaine with Buprenorphine (Bupregesic®, Neon pharmaceuticals, India) 0.3 mg intramuscularly. Group C (study group, n=25) - received 40 ml, 0.5% Lignocaine with Buprenorphine 0.3 mg in IVRA. The study was kept double blind by one anesthesiologist performing the procedure while other monitoring the patient and recording the duration and quality of analgesia. Duration of analgesia was defined as Visual Analog Scale (VAS) score of more than 25 or time to first analgesic request after deflation of tourniquet. Intravenous line was secured in contra lateral arm and lactated ringers solution infusion started. A padded double-cuff pneumatic tourniquet was then positioned around the arm, on the side to be operated. A 22G intravenous cannula was placed for drug injection in a peripheral vein, preferably over the dorsum of the hand. Now the limb was exsanguinated by elevating it to 90 degrees for three minutes followed by proximal tourniquet cuff inflation to 250 mmHg. Then a dose of 40 ml, 0.5% Lignocaine was injected, either alone or with Buprenorphine, depending upon the group, as mentioned earlier. ECG and SpO2 were monitored continuously. Blood pressure, pulse rate and respiratory rate were recorded preoperatively, immediately after the drug(s) injection and then every 5 minutes. The time onset of sensory block (by pin-prick) and that of motor block (by finger movement) was then assessed at one-minute interval. Similarly, time for complete sensory and motor blockade was also noted. After establishment of complete analgesia, distal cuff was inflated to 250 mmHg followed by deflation of proximal cuff. Throughout the procedure tourniquet pressure was monitored. Following completion of surgery tourniquet cuff was deflated with repeated deflation-reinflation technique. For this, cuff was deflated for 10 seconds and then re-inflated again for 1 minute. This sequence was repeated three times. In any case cuff was not deflated within 20 minutes of drug injection and was not kept inflated for more than 1.5 hrs. All the patients were then observed for 2 hrs postoperatively for signs of any untoward reaction. Postoperative analgesia was assessed by anesthesia resident blinded to the study, using VAS scoring at 1 hr interval. Patients were given declofenac 1 mg/kg IM whenever VAS score exceeded 25 and its total consumption in 24 hrs was recorded.Assessment of quality of block: Sensory blockade was assessed by blunt bevel pin-prick at six areas, representing smaller branches of four peripheral nerves i.e. lateral aspect of forearm for musculocutaneous nerve, dorsal 1st web space for radial nerve, index fingertip and thenar eminence for median nerve and little fingertip and hypothenar eminence for ulnar nerve. Sensory blockade was graded as: Excellent- complete anesthesia Good- complete anesthesia (touch sensation may be preserved, but not to pin-prick) Fair- adequate anesthesia with slight discomfort, tolerable without any supplementation. Poor- inadequate anesthesia requiring supplementation either systemic analgesics or general anesthesia with endotracheal intubation. Motor blockade was assessed by fine finger movement and was graded as: Excellent- completely limp. Good- minor movement of fingers. Fair- weak grip strength. Poor- good grip strength and movement of fingers. Assessment of postoperative pain was done on a Linear Visual Analog Scale (VAS) graded from 0 mm (no pain) to 100 mm (unbearable pain). Assessment of quality of block: Sensory blockade was assessed by blunt bevel pin-prick at six areas, representing smaller branches of four peripheral nerves i.e. lateral aspect of forearm for musculocutaneous nerve, dorsal 1st web space for radial nerve, index fingertip and thenar eminence for median nerve and little fingertip and hypothenar eminence for ulnar nerve. Sensory blockade was graded as: Excellent- complete anesthesia Good- complete anesthesia (touch sensation may be preserved, but not to pin-prick) Fair- adequate anesthesia with slight discomfort, tolerable without any supplementation. Poor- inadequate anesthesia requiring supplementation either systemic analgesics or general anesthesia with endotracheal intubation. Motor blockade was assessed by fine finger movement and was graded as: Excellent- completely limp. Good- minor movement of fingers. Fair- weak grip strength. Poor- good grip strength and movement of fingers. Assessment of postoperative pain was done on a Linear Visual Analog Scale (VAS) graded from 0 mm (no pain) to 100 mm (unbearable pain).Statistical analysis – All data were presented as mean±SD and number of patients. Data were analyzed using StatistiXL version 1.8 for Microsoft Excel 2003. One-way analysis of variance (ANOVA) was employed for analyzing data like onset and duration of analgesia, intra and postoperative analgesic consumption followed by Scheffe's post hoc analysis for multiple comparisons. Kruskal–Wallis test for data like quality of analgesia and VAS scores. Complication rate among groups was assessed using contingency table and Chi-Square test. P-value of less than 0.05 was considered to be significant. RESULTS Demographic data of the groups were similar for mean age, weight, and sex ratio. There was no exclusion from the study because of technical failure. There was no significant difference in duration of surgery and tourniquet time. Table 1: Patient details, times of surgery and tourniquet
There was also no difference between groups when compared for mean arterial pressure, heart rate and oxygen saturation (SpO2) at time in the intra-operative and postoperative period (P>0.05). The onset time for sensory block was longer in group C (5.0±1.0 min) as compared to group A (4.0±0.6 min) and B (4.0±0.4 min) (p=0.001) whereas there was no difference between group A and B (p=0.533). The onset of motor block did not differ between groups (p>0.05). None of the patients suffered from pain on incision in any of the groups and no patient required supplemental analgesic during surgery. The quality of sensory and motor block did not differ between groups when compared statistically (p=0.078 and p=0.088 for sensory and motor block respectively).
Table 2: Sensory and motor block details
Values as mean±SD or number. SB = sensory block, MB = motor block. E,G,F,P as excellent, good, fair and poor respectively
Table 3 shows duration of analgesia and analgesic consumption. In all the patients in group A, analgesic duration did not last beyond one hour (42±12 min). In group B mean analgesic duration was 420±36 minutes, majority of patients experienced analgesia between 6 and 8 hours. In group C mean duration of analgesia was 1200±120 minutes. Consumption of declofenac was also markedly lower in group C (56.0±9.0 mg) versus 201±27.0 and 120.0±24.0 mg in group A and B respectively (p=0.001).
Table 3: Duration of analgesia and consumption of rescue analgesic in first 24 hrs
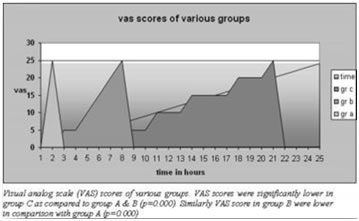
Values as mean±SD VAS scores after the tourniquet deflation for all the groups are shown in figure1. VAS scores were significantly lower in group B and C as compared to group A (p<0.0001). Similarly, there was highly significant difference between group C and B when compared statistically (p<0.0001).
All the patients were monitored for 30 minutes postoperatively and then hourly for 24 hrs thereafter for complications, if any. None of the patients in group A experienced any complication in immediate postoperative period and during subsequent 24 hours. Complication rates were significantly higher in group B patients (p=0.002). In group B a total of 18 patients had nausea and vomiting (11 and 7 respectively) whereas 8 patients had sedation, limited only to drowsiness. In contrast only two patients had single episode of nausea/vomiting while one patient had sedation in group C.
Table 4: Side effects amongst groups
Values as numbers
DISCUSSION The main finding of our study was that addition of Buprenorphine markedly prolongs duration of postoperative analgesia without causing systemic side effects. This was associated with four-fold decrease in analgesic consumption in the study group. IVRA is a preferred technique for regional anesthesia for upper extremity surgery due to ease of application, safety and low failure rate. Inability to provide effective postoperative analgesia remains one of the major disadvantages of IVRA. Ligocaine 0.5%–1% is one of the most commonly used local anesthetic for IVRA 6,7,8,9.Groups in this study did not differ when compared for demographic and hemodynamic data. Buprenorphine did not have any effect on onset or intensity of tourniquet pain which was not the major concern in our study probably as a result of using double-cuff tourniquet technique. Tsai YC et al compared EMLA cream, subcutaneous ring anesthesia and double cuff technique in the prevention of tourniquet pain and concluded double cuff technique to be most effective 13,14.In our study addition of Buprenorphine to Lignocaine in IVRA did not affect onset of motor blockade but slight prolongation of sensory block onset time was observed which is not clinically significant. This finding reported by other workers as well that addition of opioids delays sensory block time 15.Buprenorphine is a synthetic partial µ-receptor agonist, derived from thebain, one of the opioid alkaloid. It has rapid onset and prolonged duration of action. It is 25-40 times more potent than morphine on parenteral administration. It is potentially safe in conditions of over dosage due to its bell-shaped dose response curve and has a low abuse potential 16. Moreover, Yuri A. Kolesnikov, Igor Chereshnev and Gavril W. Pasternak have reported analgesic synergy between Burepnorphine and Lignocaine 17. In fact, the duration of the response from the Lignocaine/Buprenorphine combination exceeded that seen with any of the other opioids tested. Addition of Buprenorphine to Lignocaine in IVRA resulted in significant prolongation of analgesia. This was also associated with four-fold decrease in analgesic consumption in the postoperative period. Candido KD et al used Buprenorphine in brachial plexus block and reported marked prolongation of analgesia extending up to 30 hours in majority of patients, further endorsing the existence peripheral opioid antinociception 18. Similar findings are noted when Buprenorphine was added to local anesthetics in central neuraxial blocks 19,20,21. Scott S. Reuben et al have reported dose-dependent increase in duration of analgesia with meperidine in IVRA 22. Contrary to the traditional view that opioid antinociception takes place exclusively within central nervous system, there are peripheral opioid receptors that mediate analgesia, when activated by exogenous opioid agonists applied in the vicinity. This understanding of the concept of peripheral opioid receptors in sensory afferent neurons have emerged from a series of studies in animals as well humans. Research trial by Christoph Stein reveals small, systemically inactive doses of exogenous opioids administered in the vicinity of peripheral-nerve terminals has beneficial analgesic effects 4,5. This concept has already been exploited in regional anesthesia like brachial plexus blocks with much promise. Such results can also be duplicated in IVRA to alleviate its one of the major disadvantages i.e. lack of postoperative analgesia. To conclude, this study demonstrates that addition of Buprenorphine to Lignocaine for IVRA significantly prolongs the duration of postoperative analgesia possibly through peripheral mechanism while causing minimal systemic side effects. This finding also correlates with almost four-fold decrease in postoperative analgesic consumption. REFERENCES
|
|
 Home
Home