Official Journals By StatPerson Publication
|
Table of Content - Volume 12 Issue 1 -October 2019
Comparative efficacy and safety of intra-nasal dexmedetomidine and oral midazolam as pre medication in children undergoing minor elective surgical procedures: Results of a randomized, double blind trial
Shilpi Yadav1*, Vikram Vardhan2, Poonam Sangamlal Gupta3, EK Ramdas4
1Assistant Professor, 2Professor, 3Junior Resident, Department of Anesthesia, Critical Care and Pain, DY Patil University School of Medicine, Nerul, Navi Mumbai, Maharashtra, INDIA. 4Senior Consultant and HOD, Baby Memorial Hospital, Calicut, Kerala, INDIA. Email: vikramg@email.com
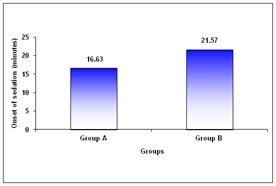
Abstract Objective: To compare efficacy and safety of intra-nasal dexmedetomidine and oral midazolam as pre medication in children undergoing minor elective surgical procedures. Material And Methods: In this randomized, double blind, trial children (1-8 years) with American Society of Anaesthesiologist (ASA) grade I and II were given intranasal dexmedetomidine 0.5 µg/kg or oral midazolam, 0.5 mg/kg and evaluated for vitals parameters and SpO2 every 15 minutes until transfer to the operation room (45 minutes). Sedation status and anxiety was assessed every 15 minutes. Onset of sedation was also compared between two groups. Results: A total of 60 children were enrolled. There was no difference in mean age ( p=0.569), weight (p=0.680) or gender (p=0.436) between two groups. At baseline and other time points there was no difference in blood pressure between two groups (p>0.05 at all-time points). Mean heart rate and oxygen saturation at all-time points was comparable (p>0.050). Onset of sedation was significantly faster with intranasal dexmedetomidine (16.63 ± 5.70 minutes) than oral midazolam (21.57 ± 8.81 minutes) (p=0.013). Mean sedation score was significantly low in intranasal dexmedetomidine than oral midazolam at 15 minutes interval (p=0.018) with no difference at 30 minutes (p=0.062) and 45 minutes (p=0.843). Mean anxiety score was significantly low in intranasal dexmedetomidine compared to oral midazolam at 15 (p<0.001) and 30 minutes (p=0.031) with no difference at 45 minutes interval (p=0.332). Conclusion: In children, intranasal dexmedetomidine is as effective as oral midazolam in terms of preoperative anxiolysis and sedation without significant difference in hemodynamic stability. Key Words: Efficacy, intra-nasal dexmedetomidine, oral midazolam, safety
INTRODUCTION Preoperative anxiety is known to prolong induction of anaesthesia and lead to new-onset maladaptive behavior in the postoperative period.1 In children, it is associated with adverse outcomes, requiring treatment with sedative premedication. About 70% of all children exhibit significant stress and anxiety before surgery2 because of perception of discomfort or harm, being separated from parents, unknown and strange environment, uncertainty of what is acceptable behavior, and threat of losing control and autonomy.3 Extreme anxiety and stress before surgery may result in negative postoperative sequelae such as emergence delirium, maladaptive behavior, and increased postoperative pain.2,4,5 Emergence delirium occurs in 12–18% of all children undergoing anaesthesia and surgery, and it is showed that it is directly related to the level of preoperative anxiety prior to surgery.4Moreover, preoperative anxiety can lead to maladaptive behaviors such as postoperative general anxiety, night time crying, enuresis, separation anxiety, apathy, withdrawal, and temper tantrums.2 It has been postulated that, increased preoperative anxiety in children is associated with postoperative pain and may hinder recovery.5 Preoperative anxiety activates stress responses, resulting in significantly increased levels of steroids and susceptibility to postoperative infections.3 Patient/parental satisfaction is a major anaesthesia outcome which is heavily dependent on the separation phase. A crying, upset child can leave parents with a feeling of dissatisfaction with the anaesthesia process.3 To minimize distress in the operating room environment and to facilitate a smooth induction is one of the challenges for pediatric anaesthesiologists.6 This is often accomplished by a multimodal approach consisting sedative drugs, parental presence, play therapy, familiar environment and effective pain therapy is necessary to reduce preoperative anxiety.7 There are three major preoperative modalities for the reduction of anxiety in children: behavioral preparation programs of various kinds, parental presence during induction of anaesthesia, and sedative premedication.8,9 The most commonly used premedications in children are midazolam and clonidine.7 The use of preoperative midazolam in children is associated with reduced anxiety in both the children undergoing surgery and reduced postoperative behavioral changes. Parents of children who received midazolam are more satisfied with the surgical experience. Preoperative medication such as clonidine reduces preoperative anxiety and postoperative pain. The use of midazolam results in antegrade amnesia that is beneficial for the recovery of the child. Midazolam is currently the most commonly used sedative drug for premedication in children.10 It has been attributed several beneficial effects such as anxiolysis, amnesia and rapid onset of sedation;11 however, an increased incidence of adverse postoperative behavior changes,12 hiccups13 and paradoxical reactions14 has also been observed. Alternatively, premedication with clonidine, although less popular, has been shown to reliably produce preoperative sedation and anxiolysis in children;15 furthermore, it has analgesic properties,16 decreases volatile anaesthetic requirements17 and improves perioperative hemodynamic stability.18 Dexmedetomidine, an α2-receptor agonist, provides sedation and analgesia which makes it a potentially useful anaesthetic premedication.19 It has more promising pharmacokinetics properties as compared to clonidine.20 Intranasal medication administration is a practical option for pediatric patients as a noninvasive alternative to the intravenous route. Its bioavailability is 81.8% (72.6–92.1%) when administered via mucosa.20,21 Atomized spray versus droplet delivery is noted to be superior in the literature due to better nasal distribution and absorption. In the areas of pain control and pre- and intra-operative sedation, inhaled medications have shown similar efficacy to intravenous options regarding time to onset of desired effect. More invasive routes of delivery, such as intramuscular, subcutaneous, intravenous (IV), or intraosseous, provide optimal drug delivery but often cause associated pain and anxiety; therefore, an alternative route of administration is often desired in the pediatric patient population.22 Effects of intranasal dexmedetomidine have been studied in children6,19,23 in smaller studies. More comparative evidence is needed to establish for wide use of intranasal dexmedetomidine as pre-anaesthestic medication. OBJECTIVES The objectives of the present study were to compare the efficacy and safety of intra-nasal dexmedetomidine and oral midazolam when given as pre medication in children undergoing minor elective surgical procedures
MATERIAL AND METHODS This randomized, double blind, controlled trial was conducted in the Department of Anaesthesiology, Baby Memorial Hospital, Calicut from March 2012 to February 2013.Children of either sex with age between one to eight years posted for elective minor surgery under American Society of Anaesthesiologist (ASA) grade I and II were enrolled. Patients with known allergy or hypersensitivity to any of the study medicines or its constituent, any organ dysfunction, cardiac arrhythmia, congenital heart disease or with mental retardation were excluded from the study. After enrollment children were randomized into two groups by lottery method. A thorough pre-anaesthetic evaluation was performed by taking history and clinical examination. In all the patients weight, basal heart rate, respiratory rate, blood pressure and clinical signs if any were recorded. Complete blood count, urine for albumin, sugar and microscopy, electrocardiogram and chest x-ray was performed if required. All the children received intra nasal medication and oral medication. Those who receive intranasal dexmedetomidine received oral paracetamol syrup and those who received intra nasal saline also received oral midazolam with oral paracetamol syrup. Children in group A received intranasal dexmedetomidine 0.5 µg/kg with oral paracetamol syrup 20 mg/kg. Intranasal dexmedetomidine was prepared from the 100 µg/mL parenteral preparation in 1 mL syringe and 0.9% saline was added to make a final volume of 0.4 mL. Children in group B received oral midazolam, 0.5 mg/kg, up to a maximum 15 mg (5 mg/mL parenteral preparation) in 20 mg/kg oral paracetamol syrup and 0.4 mL intranasal normal saline. Both the drugs were administered 45 minutes before induction of anaesthesia. All study drugs were prepared by an independent investigator not involved in the observation or administration of anaesthesia for the children. Observers and attending anaesthesiologists were blinded to the study drug given. Children had premedication in the preoperative holding area. Baseline heart rate (HR), oxygen saturation (Spo2), and blood pressure were measured before administration of drugs. Intranasal drug was instilled into both nostrils using an insulin syringe with the child in the recumbent position. Children were evaluation for vitals parameters including heart rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure and SpO2 were assessed by a blinded observer every 15 minutes until transfer to the operation room. Sedation status was assessed by a blinded observer every 15 minutes with a five point sedation scale-19 1 = Barely arousable (full sleep); 2 = Eyes closed (Light sleep); 3 = Eyes opened but looks drowsy; 4 = Awake; 5 = Agitated. Anxiety was evaluated every 15 minutes with a four point anxiety score-19 1 = Calm and sleepy; 2 = Apprehensive but withdrawn from surroundings; 3 = Crying; 4 = Agitated and difficult to control. Mode of induction (intravenous versus inhalation) was decided by the attending anaesthesiologist. The airway was maintained with a facemask or laryngeal mask airway throughout the operation. Anaesthesia was maintained with sevoflurane and 60% nitrous oxide in oxygen. Regional anaesthesia was administered whenever it was appropriate. When surgery was finished, the child was placed in the recovery position and allowed to wake up naturally in the post-anaesthesia care unit (PACU). Safety was assessed by recording vital parameters pulse rate and blood pressure and oxygen saturation. Onset of sedation was also compared between two groups. Prior to the commencement of study, ethical clearance was obtained from the Institutional Ethics Committee of the hospital. The parents/guardian/caregivers were briefed about the nature of the study and their written informed consent was obtained prior to the enrolment. Statistical analysis: The data obtained were coded and entered into Microsoft excel spreadsheet. The categorical data was expressed as percentages and continuous data was expressed as mean ± standard deviation (SD). The comparison among the groups for categorical data was done using chi-square test and student ‘t’ test was used compare continuous data. A ‘p’ value of < 0.050 was considered as significant.
RESULTS A total of 60 children were enrolled in this study. Patient disposition and baseline characteristics of the study participants are shown in Figure 1 and Table 1 respectively. Figure 1: Patient disposition There was no difference in the mean age (2.93 ± 1.85 years versus 3.23 ± 2.23 years; p=0.569), weight (13.35 ± 3.45 Kgs versus 13.80 ± 4.84 Kgs; p=0.680) gender (p=0.436) between two groups. Most of the children in both groups were aged ≤ 3 years (group A 66.67 and group B 60%) However the difference was statistically not significant (p=0.361; Table 1).
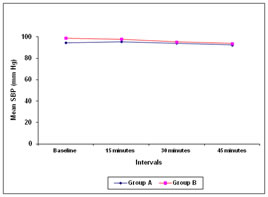
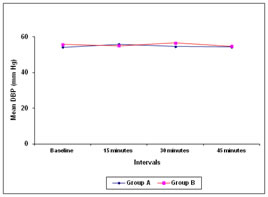
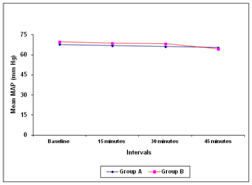
Figure 2 Figure 3 Figure 4 Figure 5 Figure 2: Comparative effects of intra-nasal dexmedetomidine and oral midazolam on mean systolic blood pressure Figure 3: Comparative effects of intra-nasal dexmedetomidine and oral midazolam on mean diastolic blood pressure Figure 4: Comparative effects of intra-nasal dexamedetomidine and oral midazolam on mean arterial pressure Figure 5: Comparison of onset of sedation in children receiving intra-nasal dexamedetomidine and oral midazolam At baseline, there was no difference in systolic as well as diastolic blood pressure between two Group A and Group B (systolic blood pressure 94.70 + 11.38 versus 99.00 + 12.13 mm Hg; p=0.162; diastolic blood pressure 54.27 + 7.23 vs 55.83 + 7.89; p=0.426). No significant difference was observed between two groups at 15 minutes (systolic blood pressure 95.67 + 11.65 vs 97.90 + 11.32 mm Hg; p=0.455; diastolic blood pressure 56.00 + 9.32 vs 55.10 + 9.56 mm Hg; p=0.713 ), 30 minutes (systolic blood pressure 94.07 + 9.30 vs 95.27 + 9.72 mm Hg; p=0.627; diastolic blood pressure 54.67 + 7.78 vs 56.60 + 8.00 mm Hg p=0.347) and 45 minutes (systolic blood pressure 92.60 +10.09 vs 93.90 + 9.95 mm Hg; p=0.617 diastolic blood pressure 54.40 + 7.29 vs 54.83 + 8.35 mm Hg p=0.831; Figure 2 and Figure 3). There was no difference in the mean arterial pressure baseline (67.74 + 8.06 vs 69.66 + 8.46 mm Hg; p=0.370), after 15 minutes (66.99 + 8.23 vs 68.60 + 8.67 mm Hg; p=0.463), 30 minutes (66.12 + 6.38 vs 68.37 + 7.68 mm Hg; p=0.223) as well as 45 minutes (65.46 + 6.13 vs 65.40 + 6.52 mm Hg; p=0.973; Figure 4) The mean heart rate as well as mean oxygen saturation at baseline, 15 minutes, 30 minutes and 45 minutes was comparable in both the groups (p>0.050; Table 2). The time required for onset of sedation was significantly less in group A (16.63 ± 5.70 minutes) compared to group B (21.57 ± 8.81 minutes) (Figure 4; p=0.013). The mean sedation score was significantly low in group A compared to group B at 15 minutes interval (1.63 ± 0.67 versus 2.10 ± 0.80; p=0.018). However no statistically significant difference was observed at 30 minutes and 45 minutes interval (p=0.062 and p=0.843 respectively). The mean anxiety score was significantly low in group A compared to group B at 15 and 30 minutes interval (1.10 ± 0.31 versus 1.70 ± 0.53; p<0.001 and 1.50 ± 0.57 versus 1.83 ± 0.59; p=0.031 respectively). However no statistically significant difference was observed at 45 minutes interval (p=0.332).
Table 1: Baseline characteristics of study participants
Table 2: Comparison of mean heart rate and oxygen saturation in children receiving intra-nasal dexmedetomidine and oral midazolam
DISCUSSION In this study, we compared the effects of intra-nasal dexmedetomidine and oral midazolam as pre medication in children before induction of anaesthesia. Sex distribution, mean age and mean weight in both the groups were comparable. In our study, mean heart rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure and SpO2 were comparable in both the groups at all the intervals including baseline (p>0.050). These findings suggest that the haemodynamic stability with intranasal dexmedetomidine is comparable to oral midazolam. A prospective randomized controlled trial conducted by O Sayal GU, et al.24 from Turkey evaluated effects of intranasal dexmedetomidine 0.5 μg/kg versus intranasal midazolam 0.5 mg/kg as premedication in 60 pre-school children aged 2-6 years in ASA I-II physical status. Heart rate was significantly higher in dexmedetomidine than midazolam at all intervals except baseline value (p<0.05). SpO2 were comparable between the groups. In the study conducted by O Sayal GU, et al.24 sedation score was significantly higher in intranasal dexmedetomidine group compared to intranasal midazolam at 15.min (p<0.05). When compared to 10 min sedation score values were significantly lower at all intervals in intranasal dexmedetomidine and intranasal midazolam (p<0.05). Parental separation score was significantly higher (p<0.05) and mask tolerance at anaesthesia induction score was significantly lower (p<0.01) in intranasal dexmedetomidine compared to intranasal midazolam. The study concluded that, intranasal 0.5 μg/kg dexmedetomidine can be an alternative to intranasal 0.5 mg/kg midazolam when used for premedication in pre-school children. In our study, intranasal dexmedetomidine required significantly lower time for the onset of sedation. Intranasal dexmedetomidine provided better sedation at the beginning up to 15 minutes compared to oral midazolam and further the sedation was comparable to that of oral midazolam. Moreover, intranasal dexmedetomidine was significantly effective in decreasing anxiety levels at 15 and 30 minutes interval while it was equally effective at 45 minutes interval compared to oral midazolam. A similar study by Schmidt et al25 compared midazolam and dexmedetomidine and showed similar levels of preoperative anxiety in both groups. The study also reported less postoperative pain in subjects given dexmedetomidine than those given midazolam. In our study, post-operative pain was not evaluated. Recently, another study by Akin A, et al.26 compared the effects of intranasal dexmedetomidine and midazolam on mask induction and preoperative sedation in pediatric patients. Ninety children classified as ASA physical status I, aged between 2 and 9, who were scheduled to undergo an elective adenotonsillectomy, were enrolled for a prospective, randomized, and double-blind controlled trial. All of the children received intranasal medication approximately 45–60 min before the induction of anesthesia. Children received either 0.2 mg/kg of intranasal midazolam, or1 µg/kg of intranasal dexmedetomidine. Satisfactory mask induction was achieved by 82.2% children receiving intranasal midazolam and 60% children receiving intranasal dexmedetomidine (p=0.01). There was no evidence of a difference between the groups in either sedation score (p=0.36) or anxiety score (p=0.56) upon separation from parents. The number of patients who required postoperative analgesia was higher in the midazolam group (p=0.045). The study concluded that, intranasal dexmedetomidine and midazolam are equally effective in decreasing anxiety upon separation from parents; however, midazolam was superior in providing satisfactory conditions during mask induction. Our study was conducted under in children undergoing elective minor surgery while the latter was conducted in children who underwent elective adenotonsillectomy only. A prospective, randomized, double-blind, controlled trial20 was conducted to evaluate whether intranasal dexmedetomidine is as effective as oral midazolam for premedication in children. Ninety-six children of ASA physical status I or II scheduled for elective minor surgery received either midazolam 0.5 mg/kg in acetaminophen syrup and intranasal placebo or intranasal dexmedetomidine 0.5 or 1 µg/kg, respectively, and acetaminophen syrup. Patients’ sedation status, behavior scores, blood pressure, heart rate, and oxygen saturation were recorded by an observer until induction of anesthesia. The results showed, no significant differences in parental separation acceptance, behavior score at induction and wake-up behavior score. When compared with midazolam, patients in dexmedetomidine 0.5 µg/kg and 1 µg/kg were significantly more sedated when they were separated from their parents (p=0.001). Patients from dexmedetomidine 1 µg/kg group were significantly more sedated at induction of anesthesia when compared with midazolam (p=0.016). The study reported more sedation with intranasal dexmedetomidine than oral midazolam, but with similar and acceptable cooperation. These findings were similar to the results of our study. In a prospective, double-blind, randomized study, Ghali M, et al.27 from Oman evaluated effects of intranasal dexmedetomidine 1 μg/kg, or oral midazolam 0.5 mg/kg at approximately 60 and 30 minutes respectively, before induction of anesthesia. The study included 120 children scheduled for adenotonsillectomy. Preoperative sedative effects, anxiety level changes, and the ease of child-parent separation were assessed. Children premedicated with intranasal dexmedetomidine achieved significantly lower sedation levels (p=0.042), lower anxiety levels (p=0.036), and easier child-parent separation (p=0.029) than oral midazolam at the time of transferring the patients to the operating room. In this study, intranasal dexmedetomidine was found to be a better choice for preanaesthetic medication than oral midazolam. Yuen et al.20 also reported that intranasal dexmedetomidine produces more sedation than oral midazolam. The behavior of the children at parental separation and at induction of anesthesia with intranasal dexmedetomidine sedation was comparable to children who received oral midazolam. Two randomized controlled trials from India reported more significant sedation with intranasal dexmedetomidine as compared to oral midazolam.6,23 On the other hand, Schmidt et al.25 found no difference in preanaesthesia levels of sedation and response to separation from parents between intranasal dexmedetomidine and oral midazolam. The differences in the results could be due to use of different scales for the assessment of sedation and anxiety level. Overall, the present study showed that, premedication with intranasal dexmedetomidine provides better sedation and anxiety levels than oral midazolam. However this study had some limitations namely, smaller sample size, children aged between one to eight years were included and the study was carried out among the children undergoing elective minor surgeries. Further studies with large sample size including other surgeries and extended age group that is up to 12 years would strengthen the efficacy of premedication with intranasal dexmedetomidine.
CONCLUSION Intranasal dexmedetomidine is as effective as oral midazolam in terms of preoperative anxiolysis and sedation. There is no significant difference in hemodynamic stability between two drugs. However further studies with large sample size are needed to confirm these findings.
REFERENCES
|
|
 Home
Home