Official Journals By StatPerson Publication
|
Table of Content - Volume 12 Issue 1 -October 2019
Evaluation of effect of low dose intravenous dexmedetomidine supplementation on sensory and motor blockade produced by intrathecal hyperbaric bupivacaine
Amol D Bhalerao1, Kumar Abhishek2*, Priti Kolarkar3, Tushar Bhavar4
1Assistant Professor, Department of Anaesthesiology, Rural Medical College, PIMS, Loni, Maharashtra, INDIA. 2Assistant Professor, Department of Anaesthesiology (Trauma Critical Care), Rajendra Institute of Medical Sciences, Jharkhand, INDIA. 3Professor, Department of Anaesthesia, DY Patil Medical College, Navi Mumbai, Maharashtra, INDIA. 4Associate Professor, Department of Anaesthesiology, Rural Medical College, PIMS, Loni, Maharashtra, INDIA. Email: amolbhalerao49@gmail.com
Abstract Background: Dexmedetomidine, a new α2 agonist, has been proven to prolong spinal anesthesia when administered through intrathecal route. The present study was aimed to evaluate the effects of intravenous dexmedetomidine on sensory and motor block characteristics, hemodynamic parameters and sedation during subarachnoid block. Materials and Methods: 80 patients with ASA grade I/ II aged 18-65 yrs undergoing infraumbilical and lower limb surgeries under spinal anaesthesia were randomized into two groups of 40 each. Group D received IV dexmedetomidine 0.5 mcg/kg bolus over 10 min by infusion pump prior to subarachnoid block (SAB), followed by an infusion of 0.5 mcg/kg/h for the duration of the surgery. Group C received similar volume of normal saline infusion. Time for the onset of sensory and motor blockade, cephalad level of analgesia and duration of analgesia were noted. Sedation scores using Ramsay sedation score (RSS) and hemodynamic parameters were assessed. Results: Onset of sensory block in group D was (1.175 ± 0.3848 min) and (1.500 ± 0.5064 min) in group C. Time for two segment regression was (132.900 ± 9.935 min) in group D and (107.751 ± 10.374 min) in group C. Time for return of modified bromage score to 0 in group D was (223 + 12.179 min) and (168.00 ± 7.579 min) in group C. Total analgesia duration was (260.125± 9.233 min) in group D and (200.000±9.199) min in group C. Conclusion: Intravenous Dexmedetomidine prolonged spinal bupivacaine sensory and motor blockade and provided satisfactory arousable sedation. It can cause transient, easily treatable bradycardia and hypotension. Key Words: Intravenous, Dexmedetomidine Supplementation, Spinal Anaesthesia
INTRODUCTION Spinal anaesthesia is a popular and common anaesthetic, technique used for lower abdominal, gynaecological, pelvic and lower extremity surgeries. Bupivacaine has been commonly used anaesthetic agent for spinal anaesthesia. Bupivacaine has long duration of action with prolonged motor block, hence appears to be appropriate for procedures lasting up to 2 hours. If the duration of surgery prolongs, it may need supplementation with intravenous anaesthetic agents or may have to be converted into general anaesthesia. Moreover, spinal anaesthesia plays pivotal role in control of intraoperative pain. Hence, researchers have used battery of drugs intrathecally like epinephrine, fentanyl, buprenorphine, midazolam, ketamine and many others as adjuvant to local anaesthetics to prolong the duration of sensory block and achieve longer perioperative analgesia.[1] Clonidine and dexmedetomidine have been used intrathecally.[2] and also intravenously to prolong the duration of spinal anaesthesia.[3-7] They produce sedation and anxiolysis by binding to presynaptic α2 receptors in locus ceruleus.[7] Postsynaptic activation in CNS inhibits sympathetic activity thus decreasing heart rate and blood pressure. At the spinal cord stimulation of α2 receptors at the substantia gelatinosa of the dorsal horn leads to inhibition of firing of the nociceptive neurons and inhibition of release of substance P contributing to their analgesic action. The most accepted mechanism of this action is by release of nitric oxide. Dexmedetomidine is more suitable adjuvant to spinal anaesthesia compared to clonidine as it has more sedative and analgesic effect due to it is 7-8 times selective α2A receptor agonist activity than clonidine. There are few studies evaluating the efficacy of dexmedetomedine in prolonging the duration of subarachnoid block and amongst them number of studies have used 1µg bolus followed by infusion.[3-4,6] Hence, present study was designed to evaluate the effect of intravenous dexmedetomedine 0.5µg/kg followed by its infusion, on the duration of spinal anaesthesia in lower abdominal and lower limb surgeries.
MATERIALS AND METHODS After obtaining approval from the Institutional Ethics Committee, this study was carried out in 80 patients of age group 18-65 years of American Society of Anaesthesiologists (ASA) physical status I or II, undergoing elective lower abdominal and lower limb surgeries under spinal anaesthesia in NKP Salve Institute medical science research centre Nagpur. In this prospective case controlled randomized double blind study, patient were randomly allocated in two groups (group C and group D) of 40 each. Randomization was done by computer generated randomization table. Group D (study group) received IV dexmedetomidine 0.5 mcg/kg bolus over 10 min by infusion pump prior to subarachnoid block (SAB), as premedication, followed by an infusion of 0.5 mcg/kg/hr for the duration of the surgery. Group C (control group) received same volume of normal saline via infusion pump. The drug solutions were prepared under sterile precautions by an anaesthesiologist who was not involved in administering and further monitoring of the patient. The anaesthesiologist performing the spinal block and monitoring the patient was unaware of the drug administered. Patients with past history of hypersensitivity to any of the test drug, diabetes, cardio-respiratory, hepatic and renal disease, psychiatric or neurological disorder, infection at puncture site, pre-existing neurological deficits in the lower extremities, coagulation defects, pregnant patient, any opioids or sedative medications consumed a week prior to surgery, history of alcohol or drug abuse were excluded from the study. Written informed consent was obtained from all patients who fulfilled the inclusion criteria with height 150-180 cm and were willing to participate in the study. During preoperative evaluation, patients were given instructions to use a 10 point visual analogue scale (VAS), with 0 indicating no pain and 10 indicating the worst imaginable pain. After an overnight fast of 8-10hrs, patients did not receive any premedication on the day of surgery. Before induction of SAB, all patients were preloaded with Ringer lactate 10ml/kg using 18-G cannula. A single dose of dexmedetomidine 0.5µgkg-1 was administered iv in 100 ml normal saline over 10 min to group D by using infusion pump via another peripheral vein cannulation. The same amount of saline was given to the patient in the group C. After SAB, supplemental dexmedetomidine infusion was continued in group D using infusion pump at the rate of 0.5mcg/kg/hr throughout the surgery. Same amount of normal saline was administered in control group in addition to routine requirement of iv fluid. Monitoring included three lead ECG with Std. lead II, Non-invasive Blood pressure, Respiratory rate, Pulse oximetry for peripheral oxygen saturation (SpO2) and Capnography for end-tidal carbon dioxide concentration (Et-CO2). The base line Heart rate, Blood pressure, SpO2, Respiratory rate, Et-CO2 were recorded prior to premedication, after premedication, then after SAB, at every 1 minute for 5 minutes; then every 5 minutes for another 25 minutes; then every 15 minutes till the procedure is completed and every 30 min in postoperative period. Under all aseptic precautions, a lumbar puncture was performed with 25 gauge Quincke needle at L3/4 interspace with patients in lateral position through midline approach with bevel point tip upward. SAB was given with15mg of 0.5% hyperbaric bupivacaine injected after free flow of clear CSF. Patient was made to lie down supine immediately on OT table without any tilt. All patient received oxygen at 2 lit/min via binasal prongs throughout the surgery. Arterial oxygen saturation was monitored continuously by pulse oximetry. Intraoperatively fluid administration was continued with ringer lactate. After SAB, in addition to regular IV fluid supplementation, all the patients in Group D was received maintenance infusion of dexmedetomidine at rate of 0.5mcg/kg/hr and same rate of infusion of saline was administered in group C throughout the duration of surgery, in same way. Hypotension (MAP≤ 25% from baseline or systolic pressure < 90mm of Hg) was treated with inj ephedrine 6 mg IV and bolus administration of 250 ml of Ringer lactate over 10 min. Bradycardia (HR<25% from baseline or HR<50 beats/min) was treated with inj Atropine 0.6 mg IV. Respiratory depression was defined as an Et-CO2>50 mm Hg or RR <12 breaths /min. Onset of sensory anaesthesia was tested by non traumatic pinprick using 23 G hypodermic needle. Time taken for onset of sensory anaesthesia at L1 level after intrathecal injection was tested for every minute till the peak level was achieved. Peak sensory level defined as the sensory level which remains same for three reading after every 1 min of interval. Peak sensory level and time to achieve peak sensory level was recorded. Two dermatomal regressions from the maximum level and regression to L1 level was noted. Sensory blockade was assessed every minute for first 10 minute thereafter every 15 min during surgery and every 30 min postoperatively till time of 1st rescue analgesia. The time for total duration of analgesia (time from administration of SAB until the first request of rescue analgesia at VAS ≥ 3) was calculated. All duration was calculated considering the time of spinal injection as time 0. Motor blockade was determined using Modified Bromage scale. Grade 0: Free movement of legs and feet, Grade I: Knee flexion decreased but with full flexion of feet and ankles, Grade II: Unable to flex knees, flexion of ankle and feet present, Grade III: Unable to flex knee or ankle, or move toes. Motor blockade was assessed every 2 min after SAB and every 30 min in PACU. The onset of motor blockade (Time taken for motor blockade to reach Modified Bromage Scale 3) and duration of motor blockade (Regression of motor blockade to Modified Bromage scale 0) was noted. Visual Analogue Scale (VAS) was used for assessment of duration of analgesia postoperatively till request of first rescue analgesic. Total duration of analgesia was defined as time from administration of SAB until the first request of rescue analgesia at (VAS≥3). Injection diclofenac 75mg intramuscular was used as rescue analgesic. The level of sedation was evaluated both intra and post operatively every 15 min thought the study period using Ramsay sedation score till the patient was discharged from PACU. Ramsay sedation score (RSS) given below : Grade I: Anxious or restless or both. Grade II: Cooperative, orientated and tranquil. Grade III: Responding to commands. Grad IV: Brisk response to stimulus.
Grade V: Sluggish response to stimulus Grade VI: No response to stimulus Adverse effect such as hypotension, bradycardia, nausea, vomiting, shivering, urinary retention and headache were observed and treated accordingly. Sample size calculation was based on previous study.[14] The data on comparison of sensory and motor parameter was referred. The standardized effect size ranged between 0.2 to 0.6 for various parameters. An average effect size of approximately 0.65 was used to determine the sample size. For a power of 0.8 and significance level of 0.05, the estimated per group sample size was 39 per group, we included 40 patients in each group for better validation of results. All parametric data were statistically analyzed using Student’s t-test and non-parametric data analyzed using Chi‑square test and Wilcoxon rank sum test.The data was analyzed using SPSS 20.0 (SPSS Inc.) software and the significance level was set at 5%.
RESULT The two groups were statistically similar to each other with respect to age, sex, height, weight, BMI, type of surgery and duration of surgery (Table-1). Onset of sensory block at L1 in group D was (1.175 ± 0.3848 min) while it was (1.500 ± 0.5064 min) in group C. This difference was statistically significant (p-value 0.0018). There was no significant difference in onset of motor blockade, as it was (4.750 ± 0.9806 min) in group D and (4.900 ± 1.008 min) in group C (p-value 0.5018). Time for two segment regression was longer in group D (132.900 ± 9.935) than group C (107.751 ± 10.374)( p- value<0.0001). Duration of motor block was prolonged in group D (223 + 12.179 min) as compared to group C (168.00+7.579 min) (p<0.0001). In dexmedetomidine group, the time for request of first dose of analgesic was (260.125+ 9.24 min) significantly prolonged as compared to control group (200.00+9.199 min) with P-value <0.0001 (table-2). In dexmedetomidine group, 6 patients developed bradycardia within 45 min to 60 min after SAB, while none of the patient in control group. The incidence of bradycardia and requirement for atropine were significantly higher in dexmedetomidine group. However, bradycardia was transient and responded well to atropine. In dexmedetomidine group 1 patient and in control group 2 patients developed hypotension. There was no statistical significant difference in the requirement of ephedrine and IV fluid between the groups. The median of highest Ramsay sedation score was 2 in group D and 1 in group C. Dexmedetomidine provided excellent sedation during the surgery and sedation score reached normal within 30 min after stopping the drug.
Table 1: Demographic profile of patient
Table 2: Sensory and motor blockade characteristics
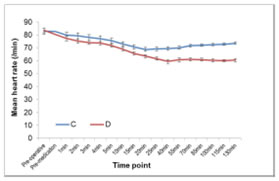
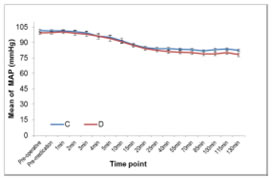
Data expressed as mean±SD. *P<0.05 statistically significant, **P<0.001 statistically highly significant. SD = Standard deviation. Data analysed by using t-test for independent samples and maximum sensory level between the groups was compared using Wilcoxon rank sum test. Intraoperative HR and MAP between the two group are shown in figure 1 and 2. Figure 1 Figure 2 Figure 1 and 2: Mean intra-operative HR and Mean MAP at each time point for two groups DISCUSSION Subarachnoid block is a common regional anaesthetic technique for sub umbilical surgical procedures. The intrathecal 0.5% hyperbaric bupivacaine is appropriate for surgeries lasting for about 120 min. To prolong the duration of analgesia and enhance the spinal anaesthetic efficacy, the adjuvants from different pharmacological classes of drugs have been studied. Adjuvants during spinal anesthesia reduce the dose requirements of local anaesthetics agents and hence their side effects[8,9] Opioids have attained an integral role as a spinal anaesthetic adjuvant, but its addition to local anaesthetic solution may lead to pruritus and respiratory depression.[10] Clonidine, α2 -adrenergic agonist has been used widely intrathecally and intravenously after subarachnoid block to prolong the sensory and motor blockade without any such adverse effects.[11] Recent studies have shown the efficacy of both intrathecal and intravenous dexmedetomidine in prolonging spinal anaesthesia. Dexmedetomidine is a more suitable adjuvant to spinal anaesthesia compared to clonidine as it has more sedative and analgesic effects due to its more selective α2 A receptor agonist activity. IV administered dexmedetomidine has been shown to produce analgesic effects by acting at both spinal and supraspinal levels. The analgesic effect primarily results from the inhibition of locus ceruleus at the brain stem. In addition, dexmedetomidine infusion may result in increased activation of α2 receptors at the spinal cord resulting in inhibition of nociceptive impulse transmission. The effect seems to be mediated through both presynaptic and the post-synaptic α2 receptors.[12,13] In our study infusion of dexmedetomidine hastens the onset of sensory block, though the onset of motor blockade was not affected. Faster onset of the sensory block may be due to α2 receptor activation induced inhibition of nociceptive impulse transmission. Also there was prolongation of duration of analgesia and motor blockade. Sedation was also provided throughout the procedure without any hemodynamic instability or any other side effects. In our study we found statistically significant difference in the onset of sensory block in group D (1.175 ± 0.3848 min) than group C (1.500 ± 0.5064min) (p 0.0018*), showing hastening effect. Harsoor et al[14] observed hastening effect on sensory block onset but Reddy et al[15] and Chandrashekharappa K et al[16] found hastening of both the onsets of motor and sensory blocks. Gupta K, Tiwari V et al[17] did not observe hastening effect on motor and sensory block as iv dexmedetomidine supplementation was started 20 mins after SAB. The maximum sensory level (mean) achieved was more in group D 6.40 (6) than in control group 7.35 (8) p< 0001. Al Mustafa et al,[3] Reddy V S et al,[15] Kaya F N et al,[18] also have reported higher level of sensory block of hyperbaric bupivacaine with intravenous dexmedetomidine supplementation. There was statistically significant longer sensory blockade in group D (260.125±9.233) than control group C (200.000±9.199) ( p <0.0001). Complete regression of motor blockade was prolonged in group D than group C. (223 + 12.179 min in group D vs 168.00 + 7.579 min in group C, P< 0.0001). Harsoor et al.[14] Al-Mustafa et al[3] and Elcicek K, et al[5] also observed prolongation of both sensory and motor blockade with intravenous supplementation of dexmedetomidine. Kaya et al[18] did not observe any effect on motor blockade duration, as they used single dose of 0.5μg/kg dexmedetomidine. The effect of clonidine on motor blockade is concentration dependant[11], there might be same explanation of this phenomenon to dexmedetomidine also. In spite of use of 0.5μg/ kg initial loading dose, motor blockade was prolonged in our study may be due to continuous infusion of dexmedetomidine. In the our study, infusion of low iv dexmedetomidine as an adjuvant to spinal bupivacaine accelerated and prolonged the sensory block and to a lesser extent prolonged the motor block of bupivacaine spinal anesthesia. Dexmedetomidine may exert its effect on sensory and motor block through the supraspinal, spinal, and peripheral action of the α2- agonist effect. [19] There may be a direct inhibition of impulse conduction in large myelinated Aa-fibers, and the 50% effective concentration of α2-agent measured approximately four-fold that needed for C fibers.[5] This may explain the lesser effect on motor block compared with sensory block observed in our study. As per literature dexmedetomidine causes significant decrease in heart rate, mean arterial blood pressure, systolic blood pressure and diastolic blood pressure. Though the observed incidence of bradycardia was higher in dexmedetomidine group but it was transient and responded to IV atropine. This decrease in the heart rate was more in group D in comparison with group C. The lower HR observed in group D could be explained by the decreased sympathetic outflow and circulating levels of catecholamines that are caused by dexmedetomidine [20,21] In this study changes in blood pressure were without significant clinical impact and hypotension could be easily managed with bolus of IV fluids and IV ephedrine. Adverse effects like hypotension, hypertension, and bradycardia were avoided by the slow infusion of dexmedetomidine, as its rapid administration might produce hypertension and reflex bradycardia due to peripheral α2B adrenoreceptor stimulation of vascular smooth muscle that can be attenuated by slow infusion over 10 or more minutes. To evaluate various doses of IV dexmedetomidine (0.25, 0.5,1 μg/kg) on ischemic pain in healthy volunteers moderate analgesia with ceiling effect at 0.5μg/kg[22]was observed. Keeping this in mind in our study we chose dose of 0.5μg/kg given over 10 min. In present study the median of highest Ramsay sedation score was 2 in group D and 1 in group C, sedation score reached normal within 30 min after stopping the drug. Dexmedetomidine affects the locus caeruleus area of the brain, which induces sedation resembling natural sleep by means of sleep modulation. Dexmedetomidine even low doses might be cause sufficient sedation, thus providing better conditions for patient and surgeon, eliminating the need for additional sedatives and hemodynamic stability was preserved. There was no respiratory depression in any patients. Respiratory rate, SpO2 and Et-CO2 remained within normal limits. Similar result was observed in previous study.[14,16,18]
CONCLUSION In conclusion, supplementation of bupivacaine spinal anesthesia with intravenous dexmedetomidine 0.5μgm/kg loading dose followed by infusion of 0.5μgm/kg/hr produced significantly longer sensory and motor block than spinal anesthesia alone. Dexmedetomidine provided excellent sedation during surgery with higher sensory level and prolongation of analgesia in post operative period significantly. However, it also prolonged the motor blockade in the early post operative period.
LIMITATION This study is carried out in ASA grade I/II patient, there is need of further study to investigate efficacy of dexmedetomidine in paediatric and medically compromised patient population.
REFERENCES
|
|
 Home
Home