Official Journals By StatPerson Publication
|
Table of Content - Volume 12 Issue 2 -November 2019
Efficacy of bupivacaine vs ropivacaine in caudal block for children: A randomized controlled trial
Natasha Gupta1*, Madiha Shadab2, Umesh Kumar Badani3
1Attending Consultant, Department of Anesthesia, Paras Hospital, Patna, INDIA. 2Senior Resident, Department of Anesthesia, Patna Medical College and Hospital, Patna, INDIA. 3Professor and Head, Department of Anesthesia, All India Institute of Medical Sciences, Patna, INDIA. Email: gupta.natasha2010@gmail.com
Abstract Background: Bupivacaine is one of the long-lasting anesthetic agents especially when given through caudal route. Similarly Ropivacaine is a newer, long-acting anesthetic agent. The efficacy of these drugs in terms of motor blockade, hemodynamic effects and adverse effects in children aged one to six years, when administered through caudal route remains a debate and thus this study was conducted to assess the efficacy of these two drugs. Methods: Prospective randomized, double blinded study was conducted among children undergoing Uro-genital, lower abdominal and lower limb surgeries in AIIMS, Patna, during month of June 2017 to April 2018. Study includes forty participants in Bupivacaine group (group A) and forty two participants in Ropivacaine group (group B). Statistical comparison of these two drugs was done using SPSS 17. Results: The mean age of participants in this study were found to be 41.45±24.72(months) and 43.78±19.96(months) in group A and group B respectively. Duration of motor blockade, haemodynamic parameters and adverse events in group A and group B were similar between these two groups. Pain score also found to be similar between these two groups. Conclusion: This study showed similar efficacy with Ropivacaine and Bupivacaine groups when administered caudally and hence both the drugs can be used for lower abdominal, urogenital and lower limb surgeries in children. Key Word: Bupivacaine, Ropivacaine, Caudal block, Motor blockade, Children
INTRODUCTION Bupivacaine and Ropivacaine have been widely used for local infiltration, epidural, brachial plexus, caudal and peripheral nerve blocks in children, since long time. In recent times, caudal block, an adjunct to the general anesthesia for lower abdominal, genital and lower limb operations is widely used. Also caudal block was found to reduce peri-operative narcotic requirement1. Though Bupivacaine is one of the most commonly used drug for spinal anesthesia, the profound myocardial depression and even cardiac arrest can occur due to accidental intravascular injection. Resuscitation from Bupivacaine induced cardiovascular collapse is difficult and may be unsuccessful. Also, motor blockade resulting from it may be a cause of distress in the post‑operative period and may lead to delayed hospital discharge2, 3. Thus search for a novel anesthetic agent is on progress. Ropivacaine is another amide local anesthetic, which has been reported to cause less motor block and less cardiovascular events than bupivacaine4, 5. Spinal anesthesia with Ropivacaine is well documented in adults6, 7 and recently, preservative free isobaric Ropivacaine (5 mg/ml) was approved for intrathecal administration for surgery8. Ropivacaine is also found to be safer for regional anesthesia in children9. The lipid solubility of Ropivacaine is less than that of Bupivacaine, which explains it’s somewhat lower potency compared with Bupivacaine10. In an equal milligram dose Ropivacaine provides a shorter duration of analgesia and a less profound motor block than Bupivacaine, especially when small concentrations are used. At the same time, Ropivacaine is an effective alternative with fewer side effects but it is found to produce less intense motor blockade than Bupivacaine and hence many anesthetists hesitate to use Ropivacaine. However, some of the studies have shown similar motor and cardiovascular effects of Bupivacaine and Ropivacaine11, 12. It is also unclear, which drug causes more duration of analgesia13, 14. Therefore this randomized comparative study was designed to evaluate the efficacy of the drugs Bupivacaine and Ropivacaine in terms of motor blockade, hemodynamic effects and adverse effects among children aged one to six years, when it is used for caudal block.
MATERIALS AND METHODS This prospective randomized, double blinded study was conducted among children undergoing Uro-genital, lower abdominal and lower limb surgeries in AIIMS, Patna during month of June 2017 to April 2018, for elective surgical procedures. Eighty two patients between age group 1-6 years, who belongs to American Society of Anesthesiologists (ASA), grade I and II with expected surgical procedure time between 20 minutes to 2 hours and fit for surgical procedure were included in the study. Patients with history of prematurity, developmental delay, increased intracranial pressure and increased intra ocular pressure were excluded from the study. Informed consent was obtained from the child’s parents, before starting the study. The study patients were randomized and divided into two groups with forty participants in Bupivacaine group (group A) and forty two participants in Ropivacaine group (group B). Patients of group A received Bupivacaine (0.25%) 1 mg/kg, group B received Ropivacaine (0.25%) 1 mg/kg. In the preoperative room, baseline recording of heart rate, respiratory rate, systolic blood pressure and activity of child were noted. Premedication was done with intravenous glycopyrrolate (0.01 mg/kg) and all procedures were performed under general anesthesia. Induction was done with intravenous propofol 3 mg/kg and intravenous atracurium 0.5 mg/kg, followed by oro‑tracheal intubation. Anesthesia was maintained with 70% of nitrous oxide in oxygen, isoflurane 0.2-0.4% and atracurium. Based on computer generated random numbers, patients received caudal block with either bupivacaine (0.25%) 1 ml/kg or ropivacaine (0.25%) 1 ml/kg in left lateral position using a 23‑gauge short bevel needle under aseptic condition. Neither sedatives nor opioids were administered intra-operatively. HR, BP, SPO2 were recorded just before and after surgical incision and every 5min thereafter till the end of surgery. The duration of motor blockade was defined as time from zero time to return of muscle tone to normal grade or ability to stand. All observed parameters of the study were recorded and subjected to statistical analysis.15 During Post operative period patients were assessed for the quality of pain relief by using Hannallah pain scale (table - 1)16, every one hourly for 8 hours. Detailed history and the observations were documented in a proforma by the principal investigator. Data was entered in Microsoft excel and data analysis was done using statistical tests like chi-square test, independent sample t test and ANOVA, wherever appropriate in Statistical Package for Social Sciences (SPSS) version 17.
OBSERVATIONS AND RESULTS In this comparative study, there were 60% male participants in Bupivacaine group and 54.8% male participants in Ropivacaine group. Female participants were 40% and 45.2% in Bupivacaine and Ropivacaine groups respectively. The p value for gender was 0.6354, which was not statistically significant. Based on ASA classification 97.5% of the participants in Bupivacaine group and 95.2% from Ropivacaine group were under ASA class I, whereas 2.5% of the participants in Bupivacaine group and 4.8% of the participants in Ropivacaine group were under ASA II class and the difference was not statistically significant. The common surgical procedure, in both the group of participants was found to be circumcision followed by Herniotomy and Urethroplasty. The p value for the surgical procedures was found to be not statistically significant (p value 0.9781).
Table 1: Characteristics of participants in each group
The mean age of participants in this study were found to be 41.45±24.72(months) and 43.78±19.96(months) in Bupivacaine group and Ropivacaine group respectively, the difference in mean age was not found to be statistically significant. The mean time duration of anaesthesia was 94.56±23.11 in Bupivacaine group and 99.13± 20.76 in Ropivacaine group. There was no statistical significance for duration of anaesthesia and mean duration of surgery in both the groups. Duration of motor blockade in group A was found to be 124.56±54.11 mins and group B was 101.47±48.53 mins but the difference between these groups were found to be statistically not significant.
Table 2: Clinical profile of participants in each group
Adverse effects encountered by participants, in Bupivacaine group were 5% of the participants had nausea and vomiting and 2.5% of the participants had urinary retention whereas, in Ropivacaine group, nausea and vomiting and pruritis was seen in 2.4% of the participants each.
Table 3: Proportion of Adverse effects in each group
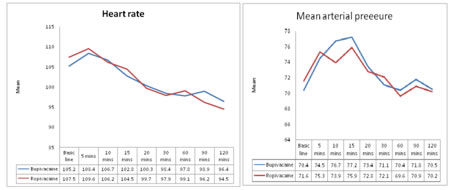
On comparison between Bupivacaine group and Ropivacaine group there was no statistical significant difference in heart rate and mean arterial pressure at various time observations. . Figure 1: Mean heart rate of participants in Bupivacaine and Ropivacaine group; Figure 2: MAP of participants in Bupivacaine and Ropivacaine group
Post operative pain score among the two groups were assessed every hourly after the surgery and it shows no significant statistical difference between Bupivacaine and Ropivacaine group.
Table 4: Mean post operative pain score of participants in both groups
DISCUSSION Post‑operative pain relief in pediatric patients needs special attention due to their inability to express the severity and type of pain. Therefore, a pragmatic practical approach of pediatric pain management has been used in recent years with the introduction of safe and effective techniques1. These days, Ropivacaine is increasingly used in the place of bupivacaine for the single shot caudal analgesia in children because it shows comparatively lesser adverse effects 1,2,4. Dobereiner et al2, Breschan et al3, Khalil et al13 and Ivani et al17 have described that efficacy of analgesia produced by ropivacaine is same as bupivacaine. In this study also we have found that efficacy of analgesia by caudally administered bupivacaine and ropivacaine were equal in both groups. In our study, the mean duration of analgesia was 94.56±23.11 min for bupivacaine and 99.13± 20.76 min in the ropivacaine group and this is comparable with the study done by Ray et al18. On the contrary Locatelli et al. found that analgesic block lasted significantly longer in patients receiving bupivacaine (P = 0.03)4. However, Khalil et al13 and Ivani et al17 didn’t support this view and found that the average duration of analgesia is same for both bupivacaine and ropivacaine. Reiz et al19 in their study proved that ropivacaine causes less cardiovascular events than bupivacaine19. In our study, hemodynamic parameters, when measured at a specific time intervals, showed no significant difference between bupivacaine group and ropivacaine group. This is comparable with the study done by Da Conceicao et al11 where the HR and arterial pressure were measured every 5 min after administration of local anesthetic and they reported no difference between the two groups. In another study conducted by Koinig et al20 on assessing the hemodynamic effects of ropivacaine and bupivacaine showed a significant decrease in mean arterial blood pressure and HR from baseline values. In this study duration of motor blockade in group A was found to be 124.56±54.11 mins and group B was 101.47±48.53 mins but the difference between these groups were found to be statistically not significant. Locatelli et al4 reported that bupivacaine produced a significant incidence of residual motor block compared with levobupivacaine or ropivacaine at wake‑up. Similarly, in another study conducted by Da Conceicao et al11 it was found that the ropivacaine group showed a shorter duration of motor block than the bupivacaine group. CONCLUSION This study showed similar efficacy in terms of duration of motor blockade and pain score of both ropivacaine and bupivacaine groups. Although the motor blockade caused by ropivacaine is less than bupivacaine, there is no statistically significant difference when compared to bupivacaine group. Also, the effect of ropivacaine and bupivacaine on hemodymamic properties like heart rate and mean arterial pressure were found to be equal among both groups.
ACKNOWLEDGEMENT I would like to thank all, who has guided me by extending their knowledge and experience right from the inception to the completion of this study. Last but not least I am thankful to my study participants, without whom, this study would not have been possible.
REFERENCES
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home