|
Table of Content - Volume 13 Issue 2 -February 2019
Comparison of propofol infusion with propofol infusion plus midazolam for induction of anaesthesia and haemodynamic stability - A randomized control trial
Bhumika Pathak1, Vaibhavi Hajariwala2*
1Assistant Professor, 2Associate Professor Pramukhswami Medical College, Shri Krishna Hospital, Karamsad, Gokul Nagar, Dist. Anand, Gujarat, INDIA.
Abstract Background: Induction and intubation are most critical events in anaesthesia, associated with major haemodynamic changes. Intravenous induction with Propofol is the most commonly used. Administration of Propofol via slow intravenous infusion helps in achieving steady state plasma concentration at effect site and makes induction rapid while using smaller dose. Concomitant use of Propofol and Midazolam can effectively reduce the dose and side effects. So we had compared the effect of Propofol infusion with Propofol infusion plus Midazolam for induction of Anaesthesia and haemodynamic stability. Materials and Methods: 100 ASA physical status I, II and III patients posted for Cancer surgeries under GA were included in this study. Patients were randomly divided into two groups. In Group P, patients were given Normal saline prior to Propofol Infusion (300 ml/min), In Group M, Inj. Midazolam 0.04mg/kg was given prior to Propofol Infusion. Loss of eyelash reflex was taken as an end point. Every minute vital parameters, total time for induction, total dosage, side effects and additional dosage of Propofol were noted. Results: Propofol dose was reduced to 65 % in Group M in comparison to Group P (p< 0.01). Group M had also more haemodynamic stability than Group P. The mean time of induction 108.82 ±5.005 seconds in Group P, 105.2 ±7.80 seconds in group M which was significantly low in Group M. Also in Group M, incidence of side effects and additional Propofol requirements were less. Conclusion: Use of Midazolam along with slow infusion of Propofol for induction of anaesthesia not only achieves the better intubating conditions but reduces the dose requirement of Propofol and decreases the side effects without producing haemodynamic instability. Key Words: Effect site, Induction, Infusion, Midazolam, Propofol,
INTRODUCTION Induction of General anaesthesia includes reversible loss of consciousness with amnesia, analgesia, muscle relaxation and inhibition of body reflexes .1 Intravenous induction is a widely used, technically easy and pleasant method of induction. Propofol provides a rapid and smooth induction within one arm- brain circulation time while maintaining airway integrity and attenuating laryngeal reflexes which helps in intubation and placement of supra-glottic airway devices even without paralysis.2 That is why Propofol is most commonly used intravenous induction agent in current anaesthesia practice. Its applications are not limited to general anaesthesia but it is also widely used for Total intravenous anaesthesia (TIVA), Monitored anaesthesia care (MAC), Conscious sedation and for ICU sedation and even for paediatric patients. Though Propofol is widely used it is not devoid of side effects, its side effects like pain on injection, hypotension, respiratory depression, epileptiform movements and Propofol infusion syndrome are all dose dependent and also dependent upon speed of injection and use of adjuvant drugs. Midazolam is an imidazobenzodiazepine which is a short acting, water soluble new benzodiazepine. Midazolam helps in achieving anxiolysis, sedation, hypnosis and anterograde amnesia.3,4 Midazolam also has anticonvulsant and skeletal muscle relaxation properties. It has its own side effects like respiratory and cardiac depression. Concomitant use of more than one pharmacological agent for achieving the same goal can effectively reduce the dose of each agents and reduces the side effects.5 Administrating Propofol via slow intravenous infusion helps in achieving steady state plasma concentration at effect site, which makes intubation easy without haemodynamic instability.6 In this study, we had studied the interaction between Propofol and Midazolam and its effect on overall haemodynamic stability and adequacy for intubation when Propofol is given via slow intravenous infusion for induction rather than conventional bolus administration. We had also studied the efficacy and safety of patients following use of Propofol infusion and midazolam for induction of anaesthesia.
MATERIALS AND METHODS This was a double blinded randomized controlled study. The study was initiated after obtaining Institutional Ethical committee approval and informed consent from the study participants. Hundred American society of Anaesthesiology Physical status class I, II and III patients, of either gender, aged between 18 to 60 years who scheduled for elective cancer surgeries and who required general anaesthesia with intubation were selected and they were divided in two groups by random selection. Randomization of patients was done by sealed envelope method (labelled with numerical) provided by the personnel not taking part in anaesthesia administration. All the medications were prepared and labelled by the anaesthesia practitioner who was not involved in administration of drug and data collection. Also the data analyzer and patients were unaware of their groups. Patients with severe respiratory, cardiac, renal, hepatic diseases, uncontrolled hypertension, epilepsy, pregnant patients, patients with psychiatric illness and not able to give informed consent were excluded from the study. Patients were divided in to two groups, Group P received Normal saline(NS) 1 minute prior to 1 % Propofol which was given as slow infusion at a rate of 300ml/min[7] via Agilia Fresenius Kabi SP syringe pump and Group M received Inj. Midazolam 0.04mg/kg[8,9] one minute prior to 1% Propofol infusion at a rate of 300ml/min. In both the groups, baseline vital parameters like Electrocardiogram (ECG), heart rate, non-invasive blood pressure (NIBP), percentage saturation of haemoglobin (SpO2) and respiratory rate (RR) were noted. After inserting 18 G cannula and starting Lactated Ringer’s solution at 50 ml/hour, pre-oxygenation started and patients were given Inj. Glycopyrrolate 5mcg/kg, Inj. Fentanyl 2 mcg/kg and either NS or Midazolam in a dose of 0.04mg/kg one minute prior to Propofol infusion administration as per randomization. After starting Propofol infusion at a rate of 300ml/min, patients were asked to count the numbers to maintain verbal contact. After loss of verbal contact patients were examined for loss of eyelash reflex. Loss of eyelash reflex was taken as an end point of induction and patients were given inj. Vecuronium 0.1mg/kg and intubation was done after 3 minutes. Total time of induction and total dose of Propofol required and time of starting of Propofol infusion were noted. Vital parameters like ECG, heart rate, NIBP, RR and SpO2 were recorded at every 1-minute interval till 5 minutes after induction. Any adverse events like desaturation, arrhythmia, hypotension, pain on injection and epileptiform movements and requirement of extra dose of Propofol for intubation were also recorded. Statistical analysis was done by using SPSS version 15 computer software. All data were expressed as mean ± 2 standard deviations. Descriptive analysis and calculated ‘Z test ‘were used to prove the statistical significance at 95% and 99% confidence limit.
RESULTS In the present study, total 100 patients were included and they were divided into 2 groups. In group P, 36 patients were males and 14 patients were females and their mean weight was 61.92 Kg. In group M, 35 male patients and 16 female patients were studied and their mean weight was 65. 26kg.Mean duration for the cancer surgeries in Group P was 176.32 minutes whereas it was 182.18 minutes in Group M. In Group P, mean dose of Propofol required for induction was 112.5 ± 12.87 mg and in group M, mean dose of Propofol was 81.56 ± 10.61 mg. Standard error of mean and Z values were calculated and it was suggesting that total dose requirement of Propofol was low in group M in comparison to group P. Reduction of Propofol dose requirement with Midazolam was not by chance and these values were statistically significant at 99% confidence limit (p<0.0001).
CONSORT flow diagram
Table 1: Comparison of total Propofol dose in both groups Mean ± SD
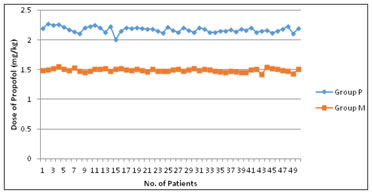
Total Propofol dose requirement was also calculated in mg/kg dose and it was found out that in Group P, Propofol requirement was 2.27 mg/kg in comparison to group M where Propofol requirement was 1.48 mg/kg which was also statistically significant at 99 % confidence limit (p<0.0001).
Table 2: Comparison of Propofol Dose (mg / kg)
In both the groups, total time of induction [ Loss of Eyelash reflex(LER)] was also recorded and compared and it was evident that the mean time of induction was 108.82 ±5.005 seconds in Group P whereas it was 105.2 ±7.80 seconds in group M. According to calculated Z –test this was statistically significant at 95 % confidence limit. Figure 1: Dose of Propofol(mg/kg) Table 3: Total time of induction (seconds)
In the study, changes in heart rate (HR), mean arterial pressure (MAP) and Percentage Saturation of Haemoglobin (SpO2) were compared for both the groups to that of the baseline values.
Table 4: Mean Arterial pressure difference (mmHg)
Table 5: Heart rate difference (HR/min) and SpO2 difference (%)
Standard error of mean and Z values were calculated for every parameter and were compared for both the groups. Results were suggesting that the Mean arterial pressure difference was statistically significant for Group M in comparison to Group P, which suggested that Midazolam potentiated the hypotensive effect of Propofol. Results for changes in heart rate and reduction in SpO2 were not clinically or statistically significant which suggests that if patients were pre-oxygenated prior to induction there was no significant fall in SpO2 and also midazolam did not potentiate the relative bradycardia response of Propofol. In the results, adverse events and requirement of additional Propofol doses were also recorded. In Group P, 2 % patients had apnea episodes where as in Group M, none of the patients were having apnea. In Group P, incidence of pain on injection was 12% where as in Group M it was 4%. In Group P, 8% patients developed excitatory movements and 4% patients required additional dose of Propofol to achieve intubation. In Group M, none of the patients had excitatory movements and 1 % patients required additional Propofol.
DISCUSSION During the entire peri-operative period, induction and intubation are the most distressing events. Until now the choice of an induction agent was based on its pharmacodynamic properties and mainly its effects on the cardiovascular system but in the light of changing time, concerns regarding depth of anaesthesia and effect on cortisol synthesis has modified this simplistic approach. Now for the choice of an induction agent, in depth knowledge of both pharmacokinetic and pharmacodynamic properties of an agent and their interaction is of utmost importance.10 Intravenous induction with Propofol is a widely used, technically easy and pleasant method of induction in adult patients. Propofol provides a rapid and smooth induction within one arm- brain circulation time while maintaining airway integrity and attenuating laryngeal reflexes which helps in intubation. However, Propofol has its own side effects like pain on injection, apnea, hypotension, relative bradycardia and infrequent excitatory movements. 2 Pharmacokinetics of the Propofol is better described by three- compartment model. 11 Propofol has short distribution half- life and long elimination half-life, so for achieving the desired effect of Propofol, steady state effect site concentration (Ce) is more important. Slow intravenous infusion of Propofol helps in maintaining steady state plasma concentration at effect site. Conventionally Propofol is given as bolus doses for induction but several studies show that Propofol can be administered at various rate but slow intravenous infusion of Propofol reduces the dose and also reduces the above-mentioned side effects.7 Propofol has a quicker onset with peak effect around 90-120 seconds whereas Midazolam has lag time of around 90 seconds for clinical effect with peak effect around 3 to 5 minutes. We had timed the administration of both the drugs so peak effect of both the drugs can be achieved simultaneously. 12 As a result of this Propofol dose requirement reduced by 65 %in Group M in comparison to group P. These results were compared to a study conducted by A. Amatya et al13 who achieved the similar results by using Midazolam as Co-induction with Propofol priming in Propofol induced anaesthesia. Our results were consistent with results of study conducted by Short et al,5 where they found out that the presence of midazolam increases the Propofol potency by 52% and other study8,14-16 who achieved the similar results and proved the synergism between two drugs. Vasant Sukumar et al17,18 studied the Ce (effect site concentration) of Propofol using target controlled infusion pump(TCI-TIVA) for induction, maintenance and recovery and they used the state entropy (SE) and neuromuscular transmission to determine depth of anaesthesia. Their results were showing that Ce and SE at the induction were 2.34±0.24µg/ml and 52±8, the average induction dose of Propofol was 1.17±0.2mg/kg. In present study, similar results were found using Propofol infusion for induction where the mean induction dose of Propofol was 1.48±0.04mg/kg (Table 2), which was less than the conventional bolus dose of 2-2.5mg/kg. In current practice, use of benzodiazepine (BDZ) is controversial especially in elderly patients19 due to its effect on post-operative cognitive dysfunction but according to meta-analysis done by Kowark et al, there is no significant risk associated with use of BDZ even in elderly patients.20 Because of the amnestic effect of the Midazolam there is a positive co-relation between the use of BDZ and reduction in the incidence of awareness during anaesthesia which is also a prime concern for anaesthesia practitioners in recent times.21 In our study, use of Midazolam potentiated the hypotensive effect of Propofol (Table 4) but it did not potentiate bradycardia response or cause respiratory depression(Table 5) and it also reduced the total time of induction (Table 3), incidence of pain on injection, excitatory movements, episodes of apnea and additional requirement of Propofol (Table 6). On subsequent follow up of the patients, we could not found any patients with post-operative cognitive dysfunction. Concomitant use of more than one agent is an age old anaesthesia practice but selection of appropriate agents, time of administration of each drug and selection of proper drug delivering system contribute in achieving desired results.
CONCLUSION Use of Midazolam along with slow infusion of Propofol for induction of anaesthesia not only achieves better intubating conditions but reduces the dose requirement of Propofol and decreases the side effects without producing haemodynamic instability. Using this combination as a part of balanced anaesthesia can also help in reducing the incidence of awareness during anaesthesia.
REFERENCES
Policy for Articles with Open Access |
|
 Home
Home