|
Table of Content - Volume 18 Issue 1 - April 2021
A randomised controlled trial comparing the efficacy of varying doses of dexmedetomidine along with 0.125% bupivacaine for caudal analgesia in children undergoing subumblical surgery
Mahesh Kumar1, M Suresh2, S Subramaniam3*
1,2Assistant Professor, 3Senior Assistant Professor, Department of Anaesthesiology, Madras Medical College, INDIA. Email: silver1432002@yahoo.co.in
Abstract Background: To assess the analgesic efficacy of varying doses of dexmedetomidine along with bupivacaine administered in the caudal space for prolonging the postoperative analgesia in children undergoing subumbilical surgeries under GA. Methods:The study included 60 children, categorized as groups A,B and C who received varying doses of dexmedetomidine, 0.5 mcg/kg , 1 mcg/kg and 2 mcg/kg respectively along with 1 ml/kg of 0.125% bupivacaine. The duration of analgesia was compared between the groups by analyzing the FLACC score. Results:The mean duration of analgesia was 298.75 min, 412.55 min and 1269.20 min in Group A,B and C respectively with statistically significant prolongation of analgesia in group C. The FLAAC was statistically significant in group C compared to groups A and B. Conclusion: Addition of 2 mcg/kg dexmedetomidine to 0.125% bupivacaine significantly prolonged the duration of analgesia with haemodynamic stability.thus minimising the requirement of rescue medications. Keywords: dexmedetomidine, pediatric patients, postoperative analgesia
INTRODUCTION Pain is an “unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage"¹. Pain experienced by infants and children often goes unrecognized, even neglected because they cannot express it2, 3. In pediatric patients, optimum pain relief is a big challenge because it is difficult to differentiate restlessness or crying due to pain from that of hunger or fear. An effective therapy to block or modify the physiological responses to painful stimulus is an essential component of pediatric anaesthesia practice.4 Regional anaesthetic techniques decreases the requirement of inhaled anaesthetics, opioids, attenuate the stress response to surgery, facilitate smooth recovery and provide good immediate postoperative analgesia with less systemic analgesic requirements.5 Among the techniques, Caudal epidural block is one of the most reliable and safe techniques for intra and postoperative analgesia in paediatric patients.5,6 The caudal blockade provides prolonged analgesia postoperatively improving patient and parent compliance, reducing complications of untreated severe pain such as tachycardia, hypertension, increased peripheral vascular resistance and neurobehavioral changes7. Prolongation of caudal analgesia using a single-shot technique has been achieved by the addition of various adjuvants such as opioids, ketamine, neostigmine, midazolam and α2 agonists8,. Many of these adjuvants have side effects like respiratory depression, vomiting, pruritus etc9,10. Among the α2 agonists clonidine and dexmedetomidine are commonly used. Clonidine has been extensively used in all types of regional anaesthetic techniques11,12,13. Dexmedetomidine is a highly selective α2 agonist with sedative and analgesic properties with minimal respiratory depression14. It has a α2/α1 selectivity ratio of (1600:1) which is eight times more potent than clonidine (200:1). It is shorter acting drug than clonidine with a distribution half- life of 9 min and elimination half- life of 2 hours16. The ideal dose of dexmedetomidine that can be given in the caudal space along with the lowest concentration of bupivacaine is still under research for implementation on a regular basis. Hence this study was undertaken to assess the analgesic efficacy of varying doses of dexmedetomidine along with 0.125% bupivacaine administered in the caudal space for prolonging the postoperative analgesia in children undergoing sub umbilical surgeries.
MATERIALS AND METHODS This Prospective randomised double blind trial was carried out at the Institute of Child Health, Rajiv Gandhi Govt General Hospital, Madras Medical College between April 2014 to August 2014. A total of 60 patients in the age group of 6 months to 6 years with physical status ASA I and ASA II, undergoing sub umbilical surgeries under general anaesthesia were included in this study. Patients with ASA III and above, infection at the site of caudal analgesia, sacral bone abnormalities, bleeding diathesis and subjects allergic to local anaesthetics were excluded from the study. Pre anesthetic evaluation was done and informed consent was obtained from the parents after explaining about the procedure. The patients were randomly allocated into three groups Group A, B and C by picking lots from a sealed envelope. All the children were premedicated with oral midazolam 0.5 mg/kg 30 minutes prior to induction of anesthesia. Routine preinduction monitors including pulseoximetry, electrocardiogram and noninvasive blood pressure monitoring were instituted. The baseline values were documented. Inhalation induction was done with oxygen 100% and sevoflurane 8% and IV cannula was secured. Ringer lactate was administered according to standard fluid administration guidelines. Inj. Fentanyl 2 mcg/kg was administered intravenously. Anaesthesia was maintained with 33% O2: 67% N2O mixture and sevoflurane 1-2%. The airway was maintained with LMA or facemask with spontaneous ventilation. After induction patients placed in lateral decubitus position and a single dose caudal block was performed under sterile conditions using 22G needle and a standard "loss of resistance" technique and the study drug given as per the allocated group. Group A received 1 ml/kg of 0.125% bupivacaine with 0.5 mcg/kg dexmedetomidine. Group B received 1 ml/kg of 0.125% bupivacaine with 1 mcg/kg dexmedetomidine. Group C received 1 ml/kg of 0.125% bupivacaine with 2 mcg/kg dexmedetomidine. The drug as per the allocated group was prepared by an anesthesia resident who was not involved in administering caudal block and data collection for the study. The caudal block was performed by another anaesthesiologist who was blinded to the drug that was injected. The surgical incision was made after 5 min of caudal placement. Duration of surgery was monitored along with heart rate, pulse oximetry, end tidal carbon dioxide with non-invasive blood pressure every 5 minutes till awakening Postoperatively, along with the vital parameters, the pain score and sedation score were assessed using FLACC score (Table 1) and RASS score(Table 2) respectively. Readings were documented at intervals of 0, 15, 30, 45, 60, 75, 90, 120,150, 180 minutes in PACU and hourly for the first 6 hours and 2nd hourly for 24 hours in ward. The time taken from the caudal placement of drug till the first recording of FLACC score more than 3 was considered as duration of analgesia. The rescue analgesic was provided with oral paracetemol 30 mg/kg when the pain score was more than 3 and the number of rescue doses were noted. The children were monitored for adverse events such as hypotension, hypertension, bradycardia, tachycardia, respiratory depression and deep sedation and treated accordingly. All the observations were recorded and all the results were analysed. Statistically data were presented as a mean ± standard deviation. A value of P < 0.05 was considered as a statistically significant. RESULTSThis study was conducted to compare the analgesic efficacy of varying doses of dexmedetomidine along with 0.125% bupivacaine administered caudally in children undergoing subumblical surgery. study enrolled 60 children undergoing subumblical surgeries in the age group of 6 months to 6 years belonging to ASA I and II physical status. The results were analysed using SPSS 17 software and a p value of less than 0.05 was considered statistically significant. When the demographic data were analysed , mean age of the three groups were compared using ANOVA tests and found to be insignificant with a p value of 0.15. Group A, B and C had a mean age of 3.16, 2.21 and 3.21 years respectively. The sex distribution between 3 groups were compared using Pearson Chisquare test. There was no significant difference between the 3 groups with a p value of 0.153.The mean weight of the patients were compared using ANOVA test and found that there was no statistical significance difference between 3 groups with a p value of 0.653. When the type of surgery and duration of surgery were analysed between 3 groups using Pearson Chisquare test and it was insignificant with p value of 0.35 .The duration of surgery between 3 groups were compared using ANOVA test. The mean duration of surgery in group A was 36.25 minutes, group B 32.05 minutes and group C 39.00 minutes with a p value of 0.4 which was not significant. The duration of analgesia (Table 3)between 3 groups were compared using ANOVA test. The mean duration of analgesia was 298.75 min in Group A, 412.55 min in Group B and 1269.20 min in Group C with a p value of 0.00 which was highly significant. The duration of analgesia were compared between groups by multiple comparisons using the Tukey HSD test. The duration of analgesia was significantly prolonged with Group C compared to Group A and B The requirement of rescue medications(Table 4) were compared between the three groups using pearsons chisquare test and it was found to be highly significant with the p value of 0.000 with group C receiving less number of rescue analgesic, followed by group B and then group A.
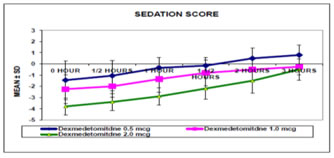
Figure 1 The sedation score (Table 5, Graph 1)was assessed using RASS score and the groups were compared using the ANOVA test and multiple comparison between groups done using Tukey HSD test. The mean sedation score was always higher in group C at 0, 30, and 60 minutes after the surgery compared to group A and group B with a p value of 0.00The heart rate at different time intervals between three groups were not significant. There was no statistical significance in the systolic and diastolic blood pressure at different time intervals in the intraoperative period The pain score was assessed using the FLACC scale and the groups were compared using pearsons chisquare test. It was found that there was a significant difference in the pain score of children in group C when compared to group A and group B at all-time intervals (Table 6).
TABLE 6: PAIN SCORE OF MORE THAN 3 AT VARIOUS TIME INTERVALS
There were two episodes of hypotension in group B and C each, as defined in the criteria which was treated with ephedrine and fluid bolus. There was one episode of bradycardia in group C as defined in the criteria which was treated with 20mcg/kg Atropine intravenously.
DISCUSSION Caudal epidural block is one of the most popular regional blocks used in children for postoperative but not an attractive choice in view of the risk of infection. So various additives to local anaesthetic solutions have been used to prolong the duration of single-shot caudal anaesthesia. Studies8,9 have reported caudal use of opioids, ketamine, midazolam, neostigmine, α2 agonists and other drugs in children to improve postoperative analgesia and prolong the duration of analgesia, but they were associated with side-effects like respiratory depression, pruritus, urinary retention and nausea/vomiting. To overcome this recent research focuses on drugs like α2 agonists. Among the α2 agonists, clonidine has been evaluated in several studies in children11 in providing prolonged duration of analgesia when combined with bupivacaine10 in different concentration.12,13 Dexmedetomidine is a highly selective α2 agonist especially for the 2A receptors and has eight times more affinity for the α2 adrenergic receptors than clonidine with much less α1 effects14,15 . Epidural dexmedetomidine has been used in the range of 1.5–2 μg/kg to prolong the duration and quality of analgesia with no optimal dose that has been recommended16. So in our study, the effect of different doses of dexmedetomidine (0.5mcg/kg, 1mcg/kg and 2mcg/kg) were given along with 0.125% bupivacaine to assess the analgesic efficacy. The search for an ideal adjuvant in caudal anaesthesia is aimed at the use of lowest concentration of local anaesthetic that provides effective analgesia with greater margin of safety. So in our study we administered 0.125% bupivacaine at 1ml//kg volume in all the three groups with varying doses of dexmedetomidine. The study groups were comparable with respect to their age, weight, type of surgery and their duration. El-Hennawy17 et al. compared the analgesic efficacy of plain bupivacaine 0.25% to that of 0.25% bupicavaine with clonidine 2 μg/kg or dexmedetomidine 2 mcg/kg and found the duration of analgesia prolonged compared to plain bupivacaine. There was no significant difference in the duration of analgesia for either of the two alpha 2 agonists. Saadawy et al. 18 showed that the mean duration of analgesia was 18.5 hrs with 1μg/kg dexmedetomidine along with bupivacaine 0.25% compared to 6.2hrs with plain bupivacaine 0.25%. In our study the mean duration of analgesia was dependent on the dose of dexmedetomidine as evidenced by significant increase in the duration of analgesia of 1269± 351.18 minutes with 2mcg/kg of dexmedetomidine compared to 298.75 ± 14.28 minutes, 412.55± 12.91 mins with 0.5 and 1mcg/kg of dexmedetomidine. This was achieved using the dilute concentration of bupivacaine 0.125% compared to other studies where the concentration of bupivacaine was 0.25%. The duration of analgesia was 21.1hrs with use of 2mcg/kg of dexmedetomidine which was the maximum compared to 4.98hrs with 0.5mcg/kg of dexmedetomidine and 6.8hrs with1mcg/kg of dexmedetomidine. The quality of analgesia were better as evidenced by the minimal requirement for rescue analgesia with 2mcg/kg of dexmedetomidine. Saadawy et al.18 and Thacacha et al. 19 showed that the incidence of agitation following sevoflurane was significantly lower with caudal use of dexmedetomidine with better quality of sleep. Neogi et al.20 found that addition of both clonidine and dexmedetomidine to ropivacaine administered caudally significantly increases the duration of analgesia. Anand et al. 21 found dexmedetomidine given caudally significantly decreases pain alon with decreased incidence of agitation. El-Hennawy et al.17 found prolonged duration of analgesia both with clonidine and dexmedetomidine given caudally without deeper levels of sedation. Ying –jun she et al.22 demonstrated addition of dexmedetomidine in caudal increased potency of levobupivacaine by increasing duration of analgesia. Xiang et al.23 found caudal dexmedetomidine significantly reduced intraop hemodynamic response to pain along with prolonged analgesia. In our study there were no children with deeper levels of sedation as measured with RASS score in all the groups. Schnaider et al.24 reported 30% decrease with 2mcg/kg of dexmedetomidine against 25% decrease in systolic blood pressure with epidural clonidine at 150mcg in adult patients undergoing upper abdominal procedures. In our study there was no significant difference in the heart rate, systolic blood pressure and diastolic blood pressure at various time intervals in the intraoperative period between the three groups probably related to the use of dexmedetomidine along with dilute concentration of bupivacaine. The incidence of adverse effects such as bradycardia was noted in one child only and hypotension occurred in 2 children that responded to ephedrine. There were no episodes of respiratory depression noted in any of the three groups reflecting the safety of dexmedetomidine for caudal analgesia in children.
CONCLUSION When comparing the groups A, B and C, group C resulted in … Prolongation of the duration of analgesia. Decreased the need for rescue analgesics . Stable hemodynamics. No significant prolonged postoperative sedation We conclude that the combination of 0.125% bupivacaine with dexmedetomidine 2 mcg/kg was better compared to 0.5 mcg/kg and 1 mcg/kg dexmedetomidine.
REFERENCES
Policy for Articles with Open Access: Authors who publish with MedPulse International Journal of Pediatrics (Print ISSN: 2579-0897) (Online ISSN: 2636-4662) agree to the following terms: Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal. Authors are permitted and encouraged to post links to their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work.
|
|
 Home
Home