|
Table of Content - Volume 19 Issue 1 - July 2021
Comparison of pre-treatment with low -dose midazolam versus low- dose etomidate for prevention of etomidate induced myoclonus and pain at intubation - A randomized controlled study
Neha Sharma1, Arti Mahajan2, Rajdeep Kour3*
1,3Lecturer, 2Assistant Professor, Department of Anaesthesia, Government Medical College, Jammu, INDIA. Email: drrajdeepkour1@gmail.com
Abstract Background: Etomidate a commonly used induction agent in anesthesia is associated with pain and episodes of myoclonus post induction. This study was designed to evaluate and compare Midazolam and low dose of Etomidate in prevention of these symptoms on injection. Methods: This prospective randomized controlled study was conducted on 90 patients allocated to three study groups. Group 1 patients received 0.015 mg/kg of Midazolam i/v diluted to 5 ml in normal saline, Group II received 0.03 mg/kg of Etomidate i/v diluted to 5 ml in normal saline and Group III received 5 ml normal saline intravenously as premedication. Five minutes after receiving the study drugs, patient was preoxygenated with 100% oxygen for 3 min along with anesthesia induction with 0.3 mg/kg etomidate injected intravenously over the period of 20-30 sec. The patients were observed for etomidate induced myoclonus and pain. Results: The incidence of myoclonus in Group II was least with 46.67% having no myoclonus as compared to group I and III where Majority of patients had grade 3 myoclonus (50%; Group I) and (46.67%; Group III). There was statistical difference between the groups in terms of myoclonus grading. Further, there was statistical difference between the groups in pain score with majority of group 1 patients having grade 3 (severe pain = 46.67%) pain and group 3 patients with 43.3% having severe pain. Group 2 on the contrary majorly reported no pain (46.6%). Conclusions: The current study indicated pre induction of etomidate in low dose as an effective strategy in prevention of EIM as compared to Midazolam.
INTRODUCTION Etomidate, an ultra-short-acting non-barbiturate imidazole derivative has been widely used in clinical practice as a hypnotic intravenous anesthetic agent since 1972. It has been widely used in general anesthesia and in rapid sequence intubation and has exhibited superior properties than propofol and thiopental in terms of seizure duration potential.1 Further, a favorable hemodynamic profile on induction with a minimal amount of blood pressure depression has been witnessed with its administration, making it an ideal choice for shock, trauma, hypovolemic patients or patients with cardiovascular disease.2 Preceding literature has reiterated the same with etomidate inducing minimal adverse effects in cardiovascular and respiratory system.3,4 It has been reported that induction dose of this drug at 0.3 mg-kg-1 causes insignificant variation in heart rate, blood pressure, systemic and pulmonary vascular resistance, stroke volume, cardiac index in adults as well as pediatric patients.4 Loss of consciousness post intra-venous administration of single bolus occurs in 15-20 seconds with a rapid recovery of 5-10 minutes. However, etomidate is also well known for some adverse effects like pain on injection, myoclonus, superficial thrombophlebitis, nausea, vomiting and adrenocortical suppression. Etomidate induced myoclonus (EIM) incidence, involuntary jerky movement, has been observed in around 50-80% of un-premedicated patients in the past4 Myoclonus may lead to patient discomfort in terms of higher risk of aspiration, regurgitation and increased intra-ocular pressure. Myoclonus prevention due to etomidate administration has been assessed with multiple drugs like (NMBA)-neuromuscular blocking agents, opioids, dexmedetomidine, gabapentin, propofol, midazolam and magnesium.5-9 Past literature emphasized Midazolam to be effective drug of treatment in Etomidate induced myoclonus (EIM) prevention.10,11 Additionally, change in etomidate injection regime itself can prove beneficial in reduction of EIM as pre-treatment with its low dose and slow administration of the dose have been associated with reduction in EIM incidence.12,13 Even though several studies have been conducted in the past to observe the association of various drugs with EIM reduction, a lacunae has been observed in comparative studies between the suggestible best alternative options. To our best knowledge, there are no reported comparative studies comparing midazolam and pre-medication with Etomidate itself for prevention of etomidate induced myoclonus (EIM) and pain on injection. We, therefore conducted the present study, to evaluate and compare the efficacies of Midazolam and Etomidate for prevention of etomidate induced myoclonus and pain on injection.
MATERIAL AND METHODS This prospective, randomized, double blind and placebo-controlled study was conducted after obtaining approval from the ethical committee of our hospital. All patients involved gave their written informed consents. Ninety patients of both genders of varying age group of 18-90 years with American Society of Anesthesiologists (ASA) physical status of I and II scheduled for various elective surgeries under general anesthesia were included in the study. Patients having allergy to any of the study drugs, history of seizure disorders, primary/secondary steroid deficiency, patients on steroid therapy, morbid obesity, MPG III and IV, pregnant and lactating patients, patients with cardiac conduction abnormalities and on antiarrhythmic drugs, sedatives or opioid therapy were excluded from the study. Patients were explained about the anesthetic technique during pre-anesthetic checkup and a written, informed consent was taken. Patients were kept nil per orally 8 hours prior to surgery. Premedication with tablet al.prazolam 0.25 mg and tablet pantoprazole 40 mg on the morning of the day of surgery with a sip of water was given. On arrival in the operation theatre electrocardiogram (ECG), pulse oximetry and non-invasive blood pressure (NIBP) were attached, and baseline parameters were recorded. A 20G intravenous (IV) cannula was secured into a vein on the dorsum of the hand and connected to ringer lactate drip. Randomization was adhered for selection of patients using a random number list into each one of the three groups to receive either of the following as a premedication. The first group (group I) patients received 0.015 mg of Midazolam i/v diluted to 5 ml in normal saline. The second group (group II) received 0.03 mg of Etomidate i/v diluted to 5 ml in normal saline. Group three (group III) received 5 ml normal saline intravenously as premedication. All drugs were prepared in 5 ml identical syringes by an independent anesthesiologist not involved further in the study. Five minutes after receiving the study drugs, patient was preoxygenated with 100% oxygen for 3 min along with anesthesia induction with 0.3 mg/kg etomidate injected intravenously over the period of 20-30 sec. The severity of etomidate induced injection pain was assessed using a 4-point scale, 0= no pain, 1= mild (pain reported only when asked), 2= moderate (pain reported without being asked or reported when asked and there were associated behavioral symptoms) and 3= severe (verbal response, grimacing, pulling the arm, tearing in the eyes). The etomidate induced myoclonus was assessed over 5 minutes after etomidate injection and its severity was graded using a four-point scale: 0= no myoclonus, 1= mild myoclonus (small movements in 1 body segment such as finger or wrist), 2= moderate (slight movements in 2 or more muscle areas such as face or shoulder) and 3= severe (intense movements in 2 or more muscle areas, sudden adduction of an extremity). The patients and the anesthesiologists involved in assessment for Etomidate induced myoclonus and etomidate injection pain were unaware of the group allocation and followed the blinding protocol. Five minutes after etomidate injection, muscle relaxation was achieved with 0.1mg/kg vecuronium and endotracheal intubation with appropriate sized endotracheal tube performed after 4 minutes. Anesthesia was maintained using 1-1.5% Isoflurane and 50% nitrous oxide and oxygen. Heart rate (HR), systolic blood pressure (SBP) and diastolic blood pressure (DBP) were evaluated during the time between injection of premedication till 10 minutes after etomidate injection at an interval of 5 minutes. The primary outcome of the study was the severity of myoclonus. The secondary outcome of the study was the severity of pain due to Etomidate injection. The data tabulations were done using MS excel 2010 and statistical analysis was done using the SPSS software 21.0 version. Statistical analysis was done using ANOVA across the three groups for comparison in continuous variables and Pearson Chi-square in case of categorical variables. The continuous data such as patient’s age, weight, heart rate, mean arterial pressure and oxygen saturation rate, ASA class were expressed as mean + standard deviation and categorical variables such as myoclonus score, pain score, sex was expressed as frequencies. P value of less than 0.05 was considered statistically significant in all the tests. \
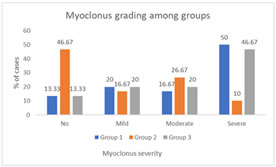
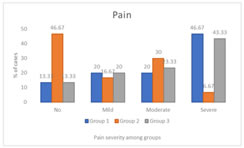
RESULTS A total of 90 patients participated in this study, with randomized selection of 30 patients each, into three groups. The demographic variables such as age, gender and weight were comparable among these groups. There was no statistically significant difference among the three groups regarding age (p value = 0.98), sex (p value = 0.48) and weight (p value = 0.87). (Table 1 and 2). Further, there was no statistically significant difference between the groups regarding the ASA grades with around 50% or more patients having ASA grade II in all three groups (Group 1=50%, group 2= 53.3% and group 3 =53.3%). (Table 3) In this study, primary outcome was observed in terms of myoclonus severity among the groups. Majority of group I patients had grade 3 myoclonus (50%) followed by grade 1 myoclonus (20%). Further, myoclonus grade 2 and grade 0 were reported among 16.67% and 13.33% patients. In group II, majority of patients had myoclonus grade 0 (46.67%) and least were reported in grade 3 myoclonus (10%). In Group III, majority of patients had myoclonus grade 3 (46.67%) followed by 20% in each grade 2 and 3 of myoclonus. There was statistical difference between the groups in terms of myoclonus grading. (p value <0.05) (Figure 1; Table 4) Further, incase of secondary outcome (severity of pain post etomidate injection); majority of group I patients had grade 3 (severe pain = 46.67%) pain followed by 20% each of the patients having moderate and mild pain. Similar results were observed in group III patients with 43.3% having severe pain followed by 23.3% patients having moderate pain, 20% patients having mild pain and 13.33% patients having no pain. On the contrary group II patients majorly reported no pain (46.6%) followed by moderate pain in 30% patients, mild pain in 16.67% patients and severe pain among 6.67% patients. There was statistical difference between the groups in pain score. (p value< 0.05) (Figure 2; Table 5) Patients during baseline (i.e., pre-surgery), post-medication, before intubation with endotracheal tube and 5 mins after putting endotracheal intubation, had similar oxygen saturation levels across the three groups. However, fluctuating variations were seen in case of heart rate and mean arterial pressure after induction. It was observed that the patients in Group I who received Midazolam had significant decreased heart rate and mean arterial pressure as compared to group II and III (p value< 0.05); Table 6, 7.
Table 1: Demographic characteristics of the patient (Age and weight)
n- number of patients, p value<0.05, SD- standard deviation, n- number of patients, p value<0.05
Table 3: Distribution of patients according to ASA grade for all the groups
n- number of patients, p value<0.05 Figure 1: Distribution of myoclonus in between the groups
Table 4: Distribution of myoclonus in between the groups
P value < 0.05
Figure 2: Comparison of pain score in between the groups
Table 5: Comparison of pain score in between the groups
P value < 0.05 Table 6: Comparison of parameters before induction among three groups
HR: Heart Rate, SPO2: Oxygen saturation, MAP: Mean arterial pressure
Table 7: Comparison of parameters before intubation among three groups
HR: Heart Rate, SPO2: Oxygen saturation, MAP: Mean arterial pressure
DISCUSSION This study evaluated the effect of Midazolam and Etomidate for prevention of etomidate induced myoclonus and pain on injection and evaluated their efficacies. The demographic variables did not imply any clinical implications in the current study as no significant differences among the groups were observed in terms of age, gender and weight. The drug etomidate, as explained earlier, has minimal impact on cardiovascular and hemodynamic changes. However, Myoclonus and pain post etomidate administration has been important complications in clinical practice of anesthesia.1,2 The etomidate administration is associated with decreased critical activity in brain and this decline occurs prior to subcortical activity control; thereby leading to myoclonus. The high prevalence of Etomidate induced myoclonus (EIM) led to various studies being conducted for control of the same.5-10,13,14 The current study indicated better results among group II i.e., patients administered (priming dose) 0.03 mg of Etomidate i/v diluted to 5 ml in normal saline as compared to Group I patients administered 0.015 mg of Midazolam i/v diluted to 5 ml in normal saline and group III with patients on placebo of normal saline solution. Around 46.67% of patients in group II had no myoclonus and no pain and the severity was also minimum in this group as compared to other two groups. The remarkable difference of Myoclonus and pain grading was significantly less in this group as compared to patients administered midazolam or normal saline with only 10% having severe myoclonus and 6.67% having severe pain in this group The preceding literature has reported utilization of various drugs in prevention of myoclonus following etomidate administration.5-9 Past literature reported low dosages of midazolam as well as Etomidate administration before anesthesia induction as effective EIM treatment modality.10-13 However, the results have been varied with some studies being more supportive of low dosage midazolam and some favoring low dosage etomidate induction to be more effective in myoclonus prevention. Nazemroaya B et al. in his study had a similar methodology to our study and administered Midazolam and low dose etomidate in the same dosage as our study (0.015 mg/kg and 0.03 mg/kg) before anesthesia induction.15 This was reiterated in similar studies by Sedighinjad et al. and Huter al using same dose of midazolam as in our study7,16 Contrary to our study, they found that low dose midazolam was associated with significantly lower incidence and intensity of myoclonus. However, our study findings in terms of etomidate priming low dose prior to anesthesia induction have been concurrent with preceding studies conducted by various researchers globally. A study done by Mullick et al. stated significantly lower rate of EIM in patients who received a bolus pre-treatment dose followed by therapeutic dose.17 Another study by Salim et al. had concurrent findings with lower episode of myoclonus among patients receiving pre-treatment as compared to other two groups but no significant difference was observed between the two groups receiving etomidate pre-treatment.18 Further, certain studies focused more on comparison of etomidate premedication with a control group without any pre-medication like Aissaoui et al., Doenicke et al. Another study by Sedighinejad et al. in 2016 did a comparative assessment of low-dose midazolam, magnesium sulfate, remifentanil, and low-dose etomidate on the prevention of myoclonus caused by etomidate for surgery.4,16,19 Their results supported our findings that low dose etomidate is effective in decreasing the prevalence and intensity of (EIM) etomidate-induced myoclonus. Our study also assessed vital signs like SPO2, mean arterial pressure and heart rate among different groups as their values can be significant in determining adverse effects of various drugs administered. Even though the parameters were comparable among three group at various stages and no adverse events occurred, significant decreased heart rate and mean arterial pressure was observed in group I after induction of Midazolam as compared to rest two groups. However, this is a normal effect of the drug midazolam and had no adverse effects in the study procedure. Previous comparative studies have reported no changes in these parameters with other drugs than the one used in our study.20 However, studies including midazolam has considered seizure duration as one of the parameters which is absent in our study.15 Our study was unique as it compared a proven effective drug midazolam and pre-treatment with etomidate and such a comparison has been rarely reported in the past. Further, the study strength was illustrated in its methodology with randomization protocol. The current study indicated pre induction of etomidate in low dose as an effective strategy as compared to other drugs prior to anesthesia in prevention of EIM. This study highlights low dose of etomidate induction prior to anesthesia during any surgery as a cost-effective treatment modality as it inhibits the unnecessary requirement of another drug introduction to the patient, further preventing any hemodynamics alteration. Even though this study has been useful with its conclusive evidence, the small sample size could be a limitation. This calls for similar studies with larger sample size for better evidence provision.
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home