|
Table of Content - Volume 19 Issue 3 - September 2021
Comparative study of magnesium sulphate and dexmedetomidine in the attenuation of pressor response to intubation and on intraoperative haemodynamic parameters in laparoscopic cholecystectomy
Prithiv Rishardhan1, Rashmee Vijay Chavan2*, Mina Bhaarathi N3, C Manisha4
1Junior Resident, 2Associate Professor, 4Ex Junior Resident, Department of Anaesthesiology, D.Y. Patil Medical College, D. Y. Patil Education Society (Deemed University), Kolhapur, 416 006, Maharashtra, INDIA. 3M.D. Anaesthesiology, Intensivist at Vijaya Group of Hospitals, Department of Anaesthesia, C.V Raman Street, Ramakrishna Nagar, Alwarthirunagar, Chennai 600087, INDIA. Email: rishi2391@gmail.com, rashmeevchavan@rediffmail.com, n.minabharathi@gmail.com, rashmeevchavan@rediffmail.com
Abstract Background: Laryngoscopy and intubation incur a reflex sympathetic discharge. Laparoscopic pneumoperitoneum usually causes a hemodynamic response characterized by tachycardia and a rise in blood pressure (BP). The main aim of this study was to compare the beneficial effects of preoperative administration of magnesium sulphate (MgSo4) and dexmedetomidine on the hemodynamic changes of intubation and laparoscopic surgery. Methodology: A randomized, prospective, double-blinded interventional comparative study was conducted on 60 patients undergoing laparoscopic cholecystectomy. Patients were randomized into 2groups, of 30 each to receive magnesium sulphate (50mg/kg(Group A)) or dexmedetomidine(1mcg/kg(Group B)) before induction. Baseline readings of systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate (HR), electrocardiography (ECG), and peripheral arterial oxygen saturation (SPO2) were recorded. Results: Among 60 patients, the percentage of patients belonging to the American society of anesthesiologists (ASA) I and II category were in group A (66.67%, 33.33%) and group B (63.33%,36.67%) respectively. Patients of both the groups were comparable on all demographic variables. Conclusion: Magnesium sulphate was better in controlling the stress response of intubation and of laparoscopic surgery. Also, it had extended analgesic effect on immediate post-operative pain which makes it better choice than dexmedetomidine to use as anesthetic adjuvant in general anesthesia in laparoscopic surgery. hemodynamic parameters (except Heart Rate). Visual analog, scale (VAS) was also lower in this group. Keywords: Intratracheal, Intubation, Laryngoscopy, Pneumoperitoneum, tachycardia.
INTRODUCTION Laryngoscopy and intubation produce a reflex sympathetic discharge which causes adverse hemodynamic changes such rise in the arterial blood pressure, heart rate, pulmonary arterial pressure and wedge capillary pressure and can even cause cardiac arrhythmias additionally these laparoscopic surgeries also incur an overall stress because of the pneumoperitoneum caused by carbon dioxide insufflation.1 Nowadays there is a vogue of opioid free anaesthesia where multiple drugs like dexamethasone, lignocaine, magnesium sulphate, paracetamol, dexmedetomidine are being used intraoperatively. Anesthesiologists are in constant search of a multimodal approach to blunt these unwanted responses. Magnesium being the fourth most common salt in the body which inhibits release of catecholamines from adrenergic nerve endings and adrenal medulla causing vasodilation and drop in BP and it serves as a good adjuvant.2 Dexmedetomidine is a selective alpha 2 agonist that stimulates the alpha-2 receptors in the lateral reticular nucleus resulting in reduced sympathetic outflow and blunting of unpleasant stimuli and hence preventing the overall hemodynamic variability.3 The main aim of this study was to compare the effect of magnesium sulphate and dexmedetomidine when given preoperatively on the stress response to laryngoscopy and laparoscopic surgery itself.
METHODOLOGY A randomized, double-blinded study of magnesium sulphate (MgSO4) and dexmedetomidine was conducted in a tertiary care hospital, between December 2018 to February 2020. The study was approved by the Institutional Ethics Committee (DYPMCK/255-A/2019/IEC). Written informed consent was obtained from patients prior to their recruitment. Subjects in the age group of 20-60 years, undergoing elective laparoscopic cholecystectomy as a part of their therapy and classified as American society of anaesthesiologists (ASA) I and ASA II were included. Patients with difficult airway, abnormal electrocardiogram (ECG), pre-existing diseases affecting neuromuscular junction and muscles, electrolyte abnormalities, seizure disorders, pre-existing cardiac, renal, hepatic, and cerebral diseases, patients in whom intubating time exceeded 20 seconds, patients in whom laparoscopic approach was transformed into an open cholecystectomy were excluded. The following formula was used to calculate the minimum sample size:
where “Zα/2” is the critical value of the Normal distribution at α/2 (e.g., for a confidence level of 95%, “α” is 0.05 and the critical value is 1.96), “Zβ” is the critical value of the normal distribution at β (e.g., for a power of 80%, β is 0.2 and the critical value is 0.84), “σ2” is the population variance (11), and “d” is the difference to detect (6).4By using the above values, the calculated minimum sample size was 52. A total of 60 patients were recruited. All enrolled patients underwent pre-anesthesia evaluation, one day prior to the surgery. Subjects were prospectively randomized by the help of computer randomization technique. The subjects were divided into 2 groups (30 patients in each group). Computer-generated randomization was performed, and the code was allocated in sealed, opaque envelopes. All subjects were administered lorazepam 1mg and omeprazole 20mg on the night prior to surgery and were nil by mouth for at least 6 hours before surgery. Patients were shifted to the operation theater with 1 mg of lorazepam, administered orally on the day of surgery. Infusion of 50 mg/kg of MgSo4 in 100ml normal saline over 20 minutes was administered prior to induction of anesthesia in one group (Group A). The other group received dexmedetomidine 1mcg/kg in 100ml normal saline over 20 minutes prior to induction of anesthesia (Group B). Once the infusion was complete, preanesthetic medications i.e., glycopyrrolate 0.004mg/kg and ondansetron 0.1mg/kg were administered. Induction of anesthesia with propofal (2mg/kg), vecuronium(0.1mg/kg), Fentanyl (2mcg/kg) was done and anesthetic gas was kept at 2%. After 3 minutes of the bag and mask ventilation adequate block was checked with a neuromuscular train of four (TOF) monitor. Intubation was executed by a senior resident with appropriate size Macintosh laryngoscope with a maximum intubating time of 20 seconds. Patients were put on controlled ventilation through a ventilator. Anesthesia was maintained by using sevoflurane at MAC of up to 1. Intraoperatively, additional analgesics (0.5mcg/kg fentanyl) or an increase in inhalational agent was done if there was greater than 10% variation from the baseline value of hemodynamic parameters. After the surgery, patients were administered neostigmine (0.05mg/kg) and glycopyrrolate (0.008mg/kg). After checking adequate reversal with a neuromuscular monitor, patients with >90% TOF ratio were extubated completely awake and monitored in post anesthesia care unit (PACU).
OBSERVATION A baseline evaluation of SBP, DBP, MBP, HR, ECG, Spo2 was obtained. After administering anesthetics, all the variables were recorded along with minimum alveolar concentration (MAC) was monitored at 1st minute,3rd minute, 5th minute and thereafter every 30 minutes until extubation. Post operatively after 2 hours the patients were assessed for pain by using a visual analog scale (VAS) and shifted to the ward based on their modified Aldrete score (MAS). Statistical Analysis Data were analysed using R version 4.0.2. Categorical variables were presented as percentages and frequency, and continuous variables were presented as mean and standard deviation (SD). Independent sample t-test was used to evaluate the difference between groups when data was normally distributed and Mann-Whitney U test was employed when the variables were not normally distributed. P<0.05 was considered statistically significant. The obtained values from the study were analyzed with TWO WAY ANOVA test. The test has employed between the variables (SBP, DBP, MBP, HR, MAC) according to group and time point to know about the correlation of the variables.
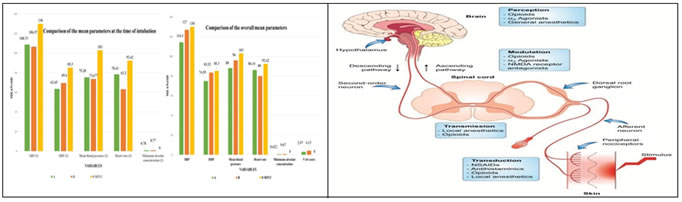
RESULTS This study enrolled 60 participants and 52.5% (n=31) of the sample were men. The mean age in Group A was 46.97±12.30 years and Group B was 46.17±15.72 years. The patients were mainly belonging to ASA- I classification in both groups. (Table 1). Table 2 represents SBP and DBP with significant values across various time points. At 1 (p<0.001), 5, 30 (p=0.04), 60 and 90 minutes (p<0.001), statistically considerable results were observed with regards to BP. Heart rate denoted significant difference in values at various time points 1 (p-value<0.0011), 3 (p-value<0.0011), 5 (p=0.00) and 30 (p=0.01). Lower heart rate was observed in group B. MAC showed significant values at 30, 60, 90 minutes (p-value<0.0011). Lower MAC was required in group A. (Table 3) Table 4 represents Systolic blood pressure (SBP),diastolic blood pressure (DBP), Mean blood pressure (MBP), heart rate (HR), MAC (Minimum alveolar concentration) at 3 minutes were observed low in group B. Figure 1 represents the summary of the research parameters. SBP, DBP, MBP, MAC, VAS was reduced in group-A as compared to group-B. HR was observed low in group-B. The TWOWAY ANOVA test has employed, the parameters (SBP, DBP, MBP, HR, MAC) were significant with P-values (P<0.05) both in the case of group and time points. This indicates that there is a significant mean difference between the parameters as well as in groups at different time points.
Table 1: Comparison of Demographic Characteristics according to groups.
Group A-50mg/kg of magnesium sulphate, Group B- dexmedetomidine 1mcg/kg, ASA- American society of anesthesiologists
Table 2: Comparative of blood pressure parameters across time points
M, Mann-Whitney-U Test; I, Independent T-test, *Statistically significant, SD - standard deviation ; MAC, minimum alveolar concentration; VAS – visual analog scale.
Table 3: Comparative of parameters across time points
M, Mann-Whitney-U Test; I, Independent T-test, *Statistically significant, SD - standard deviation; MAC, minimum alveolar concentration; VAS – visual analog scale.
Table 4: Summary of variables table at the time of intubation (3rd minute).
Group A-50mg/kg of magnesium sulphate, Group B- dexmedetomidine 1mcg/kg; SBP-systolic blood pressure; DBP-diastolic blood pressure; BP-blood pressure; SD-standard deviation; HR-heart rate; MAC-minimum alveolar concentration; min.con- minimum concentration; atm.pressussere-atmospheric pressure.
Figure 1 Figure 2 Figure 1: Graphical summary of the study variables between the groups at 3rd minute; Figure 2: Therapeutic modulation of the pain-processing pathway.
DISCUSSION Appropriate multimodal analgesia is now becoming an integral part of general anaesthesia for providing a safe balanced and opioid free anaesthesia. Giving up the old opioid-centric model, anaesthesiologists are now focusing more on nonsteroidal anti-inflammatory drugs like acetaminophen, gabapentinoids, NMDA antagonists, alpha-2-agonists, and sodium and calcium channel blocking agents.5 Such multimodal approach with desirable effects of: 1. A reduction in the requirement of opioids and its side effects (e.g., Sedation, Respiratory depression and delirium), diversion and tolerance. 2. A successful pain control policy, thereby, decreasing the associated complications of improper or inadequate pain control, pneumonia, postoperative cognitive dysfunctions and development of chronic pain and, deep vein thrombosis.5 Poor pain management hinders postoperative recovery reduces patient’s health-related quality of life, causes and adds to national health care expenditure and adds a significant personal burden. Additionally, insufficient pain relief in the postoperative period can cause and ultimately develop into chronic pain and significant postoperative cognitive dysfunction.5 Pharmacologic pain stimulus modulation along with behavioural modification is most effective, as preoperative anxiety along with development of chronic pain may lead to worsening of postoperative outcomes. This figure 2 schematically represents the blockage of pain at multiple levels by different drugs. Thus, when such drugs are combined and used as a multimodal approach the pain can significantly be blunted than with a single drug alone.6 Alpha-2 agonist have favourable pharmacological and anesthetic properties (sedation, hypnosis, anxiolysis, sympatholysis, and analgesia) that make them the most suitable adjuvants for a multimodal approach.7 Stimulation of α2-adrenoreceptors in the spinal cord and supraspinal region produces a net antinociceptive effect.5 It has been observed that clonidine as well as dexmedetomidine can reduce the requirement of opioids after the surgery, with dexmedetomidine being more effective.7 Surprisingly, the analgesic effect is more profound than acetaminophen, but it is less when compared with ketamine and NSAIDs.7 Both of these drugs are known to negatively affect hemodynamics, including their ability to cause hypotension and bradycardia. Sağıroğlu et al. compared the two different doses of dexmedetomidine (1mcg/kg and 0.5 mcg/kg) to observe the blunting of the stress response to intubation and laryngoscopy and reported that a dose of 1.0 μg/kg was much more effective than a dose of 0.5 μg/kg. We have studied the effects of dexmedetomidine in blunting the stress response with a dose of 1mcg/kg.8 Magnesium sulphate is one of the most effective analgesic adjuvant mainly for the postoperative pain.9 The analgesic property of it is mainly associated with the regulation of calcium influx into the cells, or antagonism at the NMDA receptors in the central nervous system. Additionally, it also has an anti- inflammatory effect.7 Inflammatory state may accompany with pain via peripheral or central sensitization.8 Honarmand A, Safavi M, Badiei S, Daftari-Fard N found that magnesium administered at dosages of 30 mg/kg, 40 mg/kg, and 50 mg/kg comparably attenuated the elevation in arterial pressure changes after laryngoscopy and endotracheal intubation without significant effect on HR changes. We had studied the effects of magnesium sulphate in blunting the pressor response with a dose of 50mg/kg.10 In this study we had studied the stress response with both of the above drugs not only for intubation but also for the laparoscopic pneumoperitoneum. In our study we observed that during intubation (table 3) the drop in blood pressure in either of the group was similar and was of not much significance (MAP of 73.28 and 75.68 in group A and B respectively) but the heart rate is significantly reduced better with dexmedetomidine (HR of 86.14 and 80 in group A andB respectively). Md Shahbaz Alam et al. concluded that Out of the three haemodynamic parameters (Heart rate, SBP and DBP) dexmedetomidine and magnesium sulphate are equally effective in diminishing the BP in response to intubation and laryngoscopy but, dexmedetomidine is better in controlling the heart rate.11 In our study the overall BP and MBP (table 3) corresponding to the intra-operative stress is better controlled with MgSo4 than dexmedetomidine (SBP of 114.3 and 127 in Group A and B respectively, DBP of 74.95mmhg and 83.52mmhg in group A and B respectively and a MAP of 88mmhg and 96mmhg in group A and B respectively). However, the heart rate was better controlled in people who got dexmedetomidine when compared to people who received Mgso4 (HR of 86.14 and 80 in group A and B respectively). To the best of our knowledge, this was the first study to observe the effect of hemodynamic variations due to pneumoperitoneum and post-operative analgesia with either of the drugs. Even though not very significant the requirement of MAC (0.62 and 0.67 in Group A and B respectively) is lesser in patients received MgSo4 than Dexmedetomidine. Krishna Chaitanya et al., concluded that in addition to attenuate the pressor response magnesium sulphate also reduces the requirement of anaesthetic agents in the intraoperative period.12 Post-operative pain is significantly reduced in patients received MgSo4 when compared to patients who got dexmedetomidine (VAS score of 2.97 and 4.13 in group A and group B respectively) and various Studies have showed significant reduction in postoperative pain when MgSo4 is administered intraoperatively or perioperatively.13,14,15,16,17,18,19 The Limitation of the study includes the effects of these drugs after being discharged from the post anesthesia care unit were not studied. The effects of these drugs were studied with a bolus infusion and the effects of it with a continuous infusion were not studied.
CONCLUSION Magnesium sulphate has an overall control over the hemodynamic variables (SBP, DBP, MBP) except HR, that was better controlled by dexmedetomidine, the overall requirement of MAC of sevoflurane is also decreased in Mgso4 group. VAS score was much lower in patients who received Mgso4 than patients receiving dexmedetomidine. Adverse effects or complications from any of two drugs in either of the groups was not observed.
REFERENCES
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home