|
Table of Content - Volume 20 Issue 3 - December 2021
Evaluation of efficacy of intrathecal clonidine as an adjuvant to bupivacaine in patients undergoing transurethral resection of prostate
Ragha Deepti K1, Sanjay Soloman Raj2*, Mrunalini P3
1,2Assistant Professor, 3Professor, Department of Anaesthesia, NRI Medical College and Hospital, Chinakakani, Andhra Pradesh, INDIA. Email: sanjaybonigala@gmail.com
Abstract Background: Spinal anesthesia is the standard technique for Transurethral resection of prostate (TURP) because it gives adequate sensory and motor block easily and quickly. Adjuvant intrathecal drugs are added to traditional local anaesthetics to decrease the dose of the latter and prolong the block. Clonidine is a partial alpha-2 agonist that prolongs both motor and sensory blockade when given along with local anaesthetics intrathecally. It has a well-established record of efficacy and safety. Key words: Spinal anesthesia, Clonidine, Bupivacaine, TURP.
INTRODUCTION “Divine is the task to relieve pain”- Hippocrates Relief of pain is the main challenge faced by any anaesthesiologist and this is the reason why various techniques of pain relief have been developed over ages. Spinal anaesthesia is one such technique which was introduced into clinical practice by Karl August Bier in 1898.1Spinal anaesthesia has been widely used for Transurethral resection of prostate (TURP), because it provides adequate motor and sensory blockade in addition it permits early recognition of symptoms caused by over hydration, TURP syndrome and bladder perforation.2 It is easy to perform and provides fast and effective onset of sensory and motor block. Hyperbaric bupivacaine produces a short lasting spinal anaesthesia, which may be clinically useful in ambulatory surgical procedures. It reduces surgical stress and attenuates increase in plasma catecholamine and other hormones. It gives intra as well as postoperative pain relief with full preservation of mental status and normal reflexes. Clonidine is a partial alpha-2 adrenoreceptor agonist used intrathecally as an adjuvant with a well established record of efficacy and safety.7The antinociceptive properties of clonidine indicate that it might be useful as an alternative to intrathecal opioids for postoperative analgesia,8 thus avoiding the main adverse effects, such as respiratory depression, pruritus and urinary retention. The intrathecal application of clonidine increases the duration of both sensory and motor block,9-12 as well as postoperative analgesia.5 The mechanism of analgesia with clonidine in spinal block is reported to be mediated by presynaptic (inhibition of transmitter release)13 and postsynaptic (enhancing hyperpolarization of cell membrane) effects 14,15. Clonidine enhances both sensory and motor blockade in epidural or peripheral nerve blocks using of local anaesthetics. Three possible mechanisms for this interaction have been suggested. First: clonidine blocks conduction of C and A delta fibres16 and increases potassium conductance17 in isolated neurons in vitro and intensifies conduction block of local anaesthetics.14 Second: clonidine may cause local vasoconstriction in the clinical setting, thereby reducing vascular removal of local anaesthetic surrounding the neural structures. Although clonidine and other alpha2-adrenergic agonists can vasoconstrict in high concentrations, there is little evidence for this mechanism in the concentrations used clinically. For example, plasma lidocaine concentrations are similar whether or not clonidine is combined with lidocaine for epidural anaesthesia.18 Finally, it has become evident that analgesics, whether administered systemically or with local anaesthetics, can enhance peripheral or spinal blockade. For example, intravenous or intrathecal fentanyl both enhance intrathecal lidocaine anaesthesia19,20 and the same is observed with clonidine.21, 22 However, intrathecal clonidine at the usual dose (1-2micrograms/Kg) is associated with bradycardia, relative hypotension and sedation. The aim of this double-blinded randomized study is to investigate the efficacy and adverse effects of a small dose of intrathecal clonidine added to hyperbaric bupivacaine for Transurethral surgery. Aims And Objectives: To evaluate the efficacy of intrathecal clonidine when used as an adjuvant to bupivacaine in patients undergoing TURP in terms of onset of desired sensory block, two segment regression of sensory block, duration of sensory, motor block, level of sedation, hemodynamic changes during the procedure.
METHODOLOGY The institutional ethical committee approved the protocol and written informed consent was obtained from each patient preoperatively. Sixty patients of ASA I and II undergoing transurethral resection of prostate were randomly allocated into two groups of thirty each using computer generated randomization chart. Group B received 12mg(2.4ml) of 0.5% hyperbaric bupivacaine with 0.2 ml of normal saline to make up a volume of 2.6 ml. Group C received 12mg (2.4ml) of 0.5% hyperbaric bupivacaine with 30 µg clonidine to make up a total volume of 2.6 ml. Inclusion criteria: All patients with ASA grade I and II, who gave valid written informed consent for undergoing TURP under subarachnoid block were included. Exclusion criteria: Patient refusal. ASA grade III and IV. Patients on treatment with α2 adrenergic agonists. Patients on calcium channel blockers. Patients on ACE inhibitors. Any absolute contraindication for SA. Body weight > 100Kg. Height less than 150 cm. Procedure: All patients were preloaded with 500 ml of ringer lactate solution. Under strict aseptic conditions, in right lateral position, lumbar puncture was performed at L3-L4 interspace using a 23 gauge Quincke Babcock’s spinal needle. Following the injection all patients were positioned in the lithotomy position and received 4 l/min of oxygen via face mask. The anaesthesiologist performing the block recorded the intraoperative data and a resident followed the patients postoperatively until discharge from the post anaesthesia care unit. Both the anaesthesiologist and the resident were blinded to the group to which the patient was allocated. Following parameters were observed and recorded: Assessment of sensory level: The sensory level was assessed intra- operatively by a pin prick following drug disposition using a blunt 23 gauge needle along the mid clavicular line bilaterally at 3min and for every 2 min till 20 min and every 15 min thereafter until sensory regression to S1. The onset of sensory block, highest level of sensory block and time required to achieve it, The time taken for the loss of sensory level to reach the T10 dermatome two segment regression of sensory block and regression of sensory block to S1 were noted. Assessment of motor blockade: The motor regression was assessed according to the modified Bromage scale. All durations were calculated considering the time of intrathecal injection as time zero. In case of discrepancy in the dermatomal level between the right and left side, the highest level was used for the statistical analysis. Patients were discharged from PACU after sensory regression to S1, Bromage 0, and Aldrette score of 9 were achieved. Vital parameters: The mean arterial blood pressure (MAP), heart rate (HR), and pulseoximetry were recorded at 1, 3,5,10 min and every 10 min thereafter till 1 hour after surgery followed by every 30 min till the next 1 hour. Hypotension was defined as a decrease in systolic blood pressure by 30 % from the base line, or a systolic blood pressure lower than 90 mmHg. Hypotension was treated with 6mg mephentermine IV and a bolus of 250 ml Normal saline if the blood pressure (BP) remained low. Bradycardia was defined as HR < 50 beats/ min, and was treated with 0.6 mg of intravenous atropine. The level of sedation along with respiratory depression were evaluated intra-operatively and post operatively every 15 min using the modified Wilson score. Statistical Analysis: Basic descriptive statistics have been evolved to study the central tendency and the variability among the variables. Results are expressed as the means and standard deviations, medians and ranges, or numbers and percentages. To compare and contrast and also to study the effect of clonidine between the control and the treatment groups, we implemented the TWO SAMPLE T- TEST and results are presented in the following tables. A sample size of 30 patients per group was determined through power analysis (alpha=0.05; beta= 0. 99, P = <0.05 – significant.
OBSERVATION AND RESULTS Demographic profile in both the groups was comparable in terms of age, sex, weight and the type of surgery
Table 1: ONSET OF SENSORY BLOCKADE
Values are expressed as mean±SD
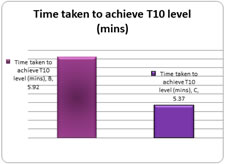
Graph 1: Onset of sensory blockade The mean time taken for onset of T10 level of sensory block to T10 level is 5.92±4.9 min in group B and is 5.37±1.81 mins in group C with a p value of 0.56 which is not statistically significant. Table 2: Time taken to attain peak sensory level
Values are expressed as mean±SD
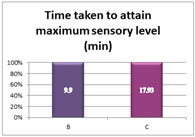
Graph 2: Time taken to attain peak sensory level The mean time taken to attain peak sensory level is 9.9±3.5 min in bupivacaine group and is 17.93±5.8 min in clonidine group with a P value- 0.00001 which is highly significant statistically. (P < 0.05) Table 3: Highest level of sensory block
Values are expressed as n (%)
Graph 3: Highest level of sensory block With regard to highest sensory level attained, 10 patients in group B (33.33%) attained highest level 0f T4-T6, 10 (33.33%) patients attained T7-T8 level and 10 (33.33%) patients attained highest level of T9-T10. In group C 23(76.67%) patients attained a maximum level of T4-T6, 4 (13.33%) patients attained T8 level and 3 (10%) patients attained a maximum sensory level of T9-T10. Table 4: Two segment regression time
Values are expressed mean±SD Graph 4: Two segment regression time The two segment regression time was considerably longer in clonidine group (81±15.1min) compared to bupivacaine group ( 52.33±9.26 min) with a statistically highly significant p value – 0.00001. (p < 0.05). Table 5: Complete sensory regression
Values are expressed as mean±SD
Graph 5: Complete sensory regression
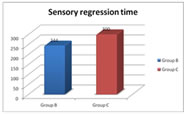
Mean time taken for complete sensory regression is 244.0±32.9 mins in bupivacaine group compared to 300.5±28 mins in clonidine group with P value – 0.00001 which is statistically highly significant. Table 6: motor regression time
Values are expressed as mean±SD
Graph 6: Motor regression time Mean time taken for complete motor regression is 234.7±30.7 mins in bupivacaine group compared to 285.5±28.1 mins in clonidine group with a P value – 0.00001 which is statistically highly significant. Table 7: reduction in mean arterial blood pressure from baseline
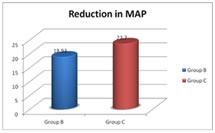
Values are expressed as mean ± SD Graph 7: Reduction in mean arterial blood pressure from baseline The mean reduction in MAP from baseline value in group B is 18.93± 8.36 mmHg compared to 23.7±8.93mmHg in group C with a P value – 0.037. Here the P value is statistically significant. Table 8: Reduction in heart rate from baseline
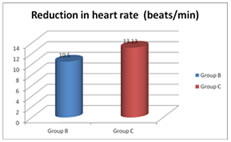
Values are expressed as mean±SD Figure 8: Reduction in heart rate from baseline The mean reduction in heart rate from baseline value in group B is 10.5±6.49 beats/min compared to 13.13±8.34 beats/min in group C with a P value – 0.18. Here the P value is statistically insignificant. Table 9: Perioperative complications
Values are expressed as numbers Out of thirty patients in 2 (6.67%) patients developed hypotension in bupivacaine group and 5 (16.67%) patients developed hypotension in clonidine group. Apart from these no other side effects were recognized.
DISCUSSION Spinal anaesthesia has been widely used for urological procedures particularly in transurethral procedures and perineal surgeries. In TURP it permits early recognition of the symptoms caused by TURP syndrome and bladder perforation.2 Review of the literature reveals very few articles on the use of intrathecal clonidine in patients undergoing TURP. The level of anaesthesia both motor and sensory is dependent on the dose, anaesthetic solution, site of injection along the neuraxis, position of the patient, baricity of local anaesthetic solution, and injection rate (0.1-.2 ml/sec) in addition to several patient factors.27 Traditional spinal anaesthesia results in higher motor and autonomic blockade, thereby it may interfere with smooth postoperative recovery in this group of geriatric patients undergoing TURP. Complications like DVT, atelectasis may occur due to restriction in bed. Hence, lower doses of local anaesthetics like Xylocaine and Bupivacaine etc, are preferable to traditional volumes. But, in procedures lasting more than one hour either due to intraopertaive complications like bleeding or large size of the gland, low dose may not achieve this goal. Hence, there has been a recent interest in using analgesic additives to spinal anaesthetics to decrease their dose, for faster recovery while maintaining adequate level of anaesthesia. However, one should take strict aseptic precautions while adding additives to the local anaesthetics. With reference to the dose response data on the clinical anaesthetic characteristics for spinal bupivacaine and with reference to the published articles on TURP using a dose of 12, 12.5 mg of intrathecal Bupivacaine (kanazi, Sethi),26,25 we have selected a dose of 12mg as optimum dose for our patients undergoing TURP. Smaller doses of intrathecal bupivacaine will reduce the number of blocked dermatomes and decrease the duration of spinal anaesthesia. Co-administration of clonidine intrathecally reduces the dose of bupivacaine and improves the quality of spinal anaesthesia. Because of the antinociceptive properties of clonidine and because it increases the duration of both sensory and motor block as well as post operative analgesia5, clonidine was chosen in preference to fentanyl or other opioids, thus avoiding the main adverse side effects such as respiratory depression, pruritus, urinary retention, nausea, vomiting and sweating. Dose response data for spinal clonidine suggests that the dose of 15-45 µg is an optimal dose for low dose spinal anaesthesia,28 Gautier and colleagues recommend 15-45 µg of clonidine as optimal dose for supplementing spinal anaesthesia.29 In keeping with this range we selected a dose of 30µg of clonidine for our study. Clonidine has been used intrathecally in doses ranging from 75-150µg30. Cardiac instability, hypotension and bradycardia are also reported with intrathecal clonidine in this range. For the selected study dose (30µg) these complications are very minimal. The patient’s characteristics and parameters were kept identical in both the groups to avoid variations in the outcome. In our study, the mean time for onset of sensory block in bupivacaine group was 5.92 mins and clonidine group was 5.37 mins. Though there is faster onset of action in the clonidine group compared to control group, this result was not statistically significant. Results of our study differed from observations of Sethi et al.,25 probably because of the higher dose of clonidine used in their study. However, this observation may not be of any clinical significance. The mean time to achieve peak sensory level in bupivacaine group compared to clonidine group was 9.9 minutes vs 17.93 minutes. In a study conducted by Grandhe et al.31, the authors observed an earlier onset of peak sensory block. This discrepancy could be due to a lower dose of bupivacaine (7.5 mg) used in this study compared to 12 mg in our study. Among both the groups, clonidine group achieved highest level of sensory block - T4-T6 23(76.67%) level. Several authors Dobrydnjov4, Gurudatta et al.,24 Sethi et al.25 using different concentrations of hyperbaric bupivacaine also had similar observations. This would help to achieve an adequate anaesthesia when complex situation prevails in TURP. However, in geriatric patients with other co-morbid conditions, higher dermatomal block may be detrimental and hence the dose of bupivacaine and clonidine may have to be tailored accordingly. The time for two-segment regression was considerably prolonged in Clonidine group (81.3min) compared to Bupivacaine group (52.3min) Which was consistent with the results of Dobrydnjov,4 Sethi et al.25 The longer two segment regression time in the clonidine group will be definitely useful in patients undergoing TURP, especially for large glands and in complicated situations and would avoid conversion to general anaesthesia. The mean time taken for sensory regression was prolonged in group C by about 50 - 60 min (244.0 mins in bupivacaine group compared to 300.5 mins in clonidine group) which was statistically highly significant. Several authors, Gurudutta et al.,24 Sethi et al.25 also concluded that there was a significant prolongation of complete sensory regression in clonidine group in their studies also as in our study. The anti - nociceptive effects of clonidine explain the prolongation of the sensory block when added as an adjuvant to local anaesthetics. It has well established synergistic effect with local anaesthetics intrathecally.3- 6Mean time taken for complete motor regression was 244.7 mins in bupivacaine group compared to 285.5 mins in clonidine group with a P value – 0.00001 which is statistically highly significant. The prolongation of motor block in our study was comparable to studies by Strebel et al. 3, and Sethi et al.25 in spite of higher volume or higher dose of clonidine. The explanation could be that alpha 2 adrenergic agonists induce cellular modification in the ventral horn of the spinal cord (motor neuron hyper polarization) and facilitate local anesthetic action. In addition due to its local vasoconstriction effect in the clinical setup.18 the above conclusions can be supported. Hence, we conclude that intrathecal clonidine potentiates the anaesthetic effects of intrathecal bupivacaine. The mean reduction in MAP from baseline (bupivacaine group is 18.93 mmHg compared to 23.7mmHg in clonidine group) was statistically significant. Even though there was a statistically significant reduction in the MAP in clonidine group compared to the control group it was clinically insignificant as none of the patients required any corrective treatment. These observations were similar to that of Sethi et al.,25 Dobrynjoy et al.4 However Agreta et al.2 in their study did not encounter hypotension as significant complication. This is probably due to usage of very low dose of bupivacaine 7mg and clonidine 25µg. Bradycardia in spinal anaesthesia is believed to result from at least two causes: Blockade of sympathetic cardio acceleratory fibres and decreased venous return to the heart. The mean reduction in heart rate from baseline value in bupivacaine group (10.5 beats/min) compared to clonidine group (13.13beats/min) was statistically insignificant. Our results were identical with that of Agreta et al.2 Dryness of mouth, a typical side effect of clonidine administration was not encountered in our patients unlike Gorth et al.13 Dobrydnjov et al.,4 in his study concluded that small doses of intrathecal clonidine are not usually associated with systemic side effects such as bradycardia, hypotension or sedation. Sedation after clonidine anaesthesia reflects systemic reabsorption and vascular redistribution to higher centres.32 In none of our subjects there was evidence of respiratory depression. According to Grubb et al.33 intrathecal clonidine provides adjunct analgesia without additional respiratory depression unlike opioids. Many studies are being conducted with clonidine as an adjuvant to bupivacaine for prolonging the post operative analgesia. The aim of these studies is to optimize the dose of intrathecal clonidine for prolonging the duration of post operative analgesia with least side effects. With the advent of bipolar cautery, TURP surgical duration has no time limit now a days and with the above findings it is evident that we can safely add 30µg clonidine as an adjuvant to hyperbaric bupivacaine for patients undergoing transurethral resection of prostate surgeries especially for large prostates and complicated surgeries. Limitation of study: A potential limitation of our study design is to use a fixed dose of clonidine -30µg added to a fixed dose of bupivacaine -12 mg. Although our study design has an adequate power, a study with larger number of subjects with varying doses of bupivacaine and clonidine will help us to draw better conclusions.
CONCLUSION Supplementation of bupivacaine spinal block with a low dose of intrathecal clonidine produces a significantly longer sensory and motor blockade than bupivacaine alone without any significant hemodynamic instability or sedation. It is an attractive alternative to opioids for prolonging spinal anesthesia. Clonidine will expand the scope and improve the reliability and efficacy of regional anaesthesia. On the basis of our present clinical comparative study, we can conclude that it is safe to add 30 µg clonidine to 12 mg of hyperbaric bupivacaine in spinal anaesthesia for elderly patients undergoing TURP. “Addition of clonidine promisingly potentiates bupivacaine spinal anaesthesia”
REFERENCES
Policy for Articles with Open Access
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home