|
Table of Content - Volume 20 Issue 3 - December 2021
A Randomized comparative study: Prophylactic use of phenylephrine infusion versus phenylephrine bolus to treat hypotension in caesarean section patients
Supriya Kulkarni1, Rashmee Vijay Chavan2*, Sandeep Kadam3, Anupama Sahasrabudhe4, Archita Patil5
1Junior Resident, 2,3,4,5Associate Professor, Department of Anaesthesiology, D. Y Patil Medical College, D Y Patil Education Society (Deemed university), Kolhapur, 416003. Maharashtra, INDIA. Email: supriyah8@gmail.com, rashmeevchavan@rediffmail.com, kadamsandeeps@gmail.com, dranuds@gmail.com, drarchita@yahoo.co.uk
Abstract Background: Subarachnoid block (SAB) is the preferred method of anaesthesia in parturient with maternal hypotension and foetal acidosis as its commonest side effects. Phenylephrine is a drug of choice to treat maternal hypotension and foetal acidosis. Therefore the study aimed to compare the effect of prophylactic phenylephrine infusion versus therapeutic bolus to treat maternal hypotension on foetal umbilical artery pH. Methods: The randomized double blinded comparative study was conducted in a tertiary care hospital. ASA I, II grade pregnant women of 20-35 years of age undergoing elective caesarean section were included (n=70). Participants were divided randomly into Group A (n=35) to receive prophylactic phenylephrine 100 mic/min for three minutes. Group B (n=35) to receive bolus phenylephrine 100 mic to treat maternal hypotension. Hemodynamic parameters were recorded after three minutes from the SAB, at one-minute intervals till delivery of the baby. Hypotension was treated with intravenous phenylephrine 100 mic. After delivery of the baby APGAR score and foetal blood pH were measured. Mann Whitney test in R-studio software (version-1.2.5001) was used for statistical analysis. Results: Systolic blood pressure, diastolic blood pressure and the mean arterial pressure were significantly high in group A (P<0.05). Heart rate was significantly low in group A at 3-5 minutes (P<0.05). Significant difference was observed in umbilical arterial pH between both the groups (P=8.317e-05). Conclusion: Prophylactic intravenous infusion of phenylephrine in women undergoing CS under SA is an effective measure in preventing hypotension and foetal acidosis. Keywords: Caesarean section; Foetal acidosis; Hypotension; Phenylephrine; spinal anaesthesia Manuscript (without Author Details)
INTRODUCTION Spinal anaesthesia (SA) is a well advocated technique used for caesarean section (CS).1 However, it is associated with maternal hypotension in 70%-80% of cases in the absence of pharmacological prophylaxis.2 Hypotension in CS may lead to minor symptoms in mother like light-headedness, nausea, vomiting dyspnoea or adverse effects in foetus like bradycardia and acidosis due to decreased uteroplacental blood flow.3 Preventive measure of SA induced hypotension at the time of CS involve, fluid preload/coload, administration of vasopressors like ephedrine, metaraminol, mephentermine and left uterine tilt.4,5 It is stated by the Cochrane review published in 2017 that no single drug or technique is ideal or gold standard in preventing subarachnoid block (SAB) induced maternal hypotension.5 Studies have been proven that preload or coload has limited efficacy in preventing maternal hypotension.6 The current practice is to use vasopressors as treatment of hypotension rather than for prophylaxis . One of the concerns to use prophylactic vasopressors is that the total amount of drug used could have adverse effects on uteroplacental blood flow. Many researchers are of the view that prophylactic vasopressors, when used soon after SA have huge advantage in reducing the incidence, frequency and severity of the hypotension.7 Amongst the vasopressors phenylephrine is recommended for obstetric patients as it has selective α-adrenergic agonist activity , rapid onset and offset of action, easy titratability and negligible transfer across placental barrier causing minimal foetal adverse effects.8, 9,10 Prophylactic phenylephrine infusion immediately after placement of SAB is the most effective preventive measure to tackle maternal hypotension.11 Various doses have been tried ranging from 25- 100 mcg/min.12 Aim of this study was to evaluate efficacy of prophylactic phenylephrine infusion in a dose of 100 mcg/min in preventing maternal hypotension and at the same time to evaluate the effect of such a large dose on the fetus.
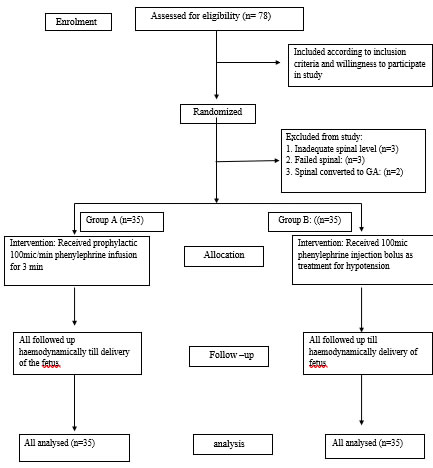
MATERIALS AND METHODSThis prospective double blinded randomized comparative study was conducted in the anaesthesiology department in a tertiary care hospital between Jan 2018 -July 2019. Approval was obtained from the Institutional Ethics and Research Committee prior to initiation of the study. Written informed consent was obtained from participants prior to the study. A total of 70 pregnant women between 20-35 years of age belonging to American Society of Anaesthesiologists (ASA) physical status II, undergoing elective caesarean section with uncomplicated singleton pregnancy were included. The pregnant women with resting blood pressure >140/90 mm Hg or heart rate < 60/min, with history of hypertension, preeclampsia, eclampsia, hyperthyroidism, hypersensitivity to anaesthetic agent or the patients having coexisting neurological, cerebrovascular, cardiovascular, renal, metabolic disorders were excluded. Parturients associated with increased risk of bleeding like low lying placenta, severe anaemia, diabetes, foetal distress and contraindications to SAB were also excluded. Patients were randomly divided by computer generated random number tables into two groups of 35 each. Group A (n=35) was the study group to receive prophylactic phenylephrine infusion. Group B (n=35) was the control group where phenylephrine bolus was used to treat maternal hypotension. (Flow chart CONSORT 2010) All patients were thoroughly clinically evaluated and investigated on the day before surgery. Patients were premedicated with tab ranitidine 150 mg orally the night before and on the morning of the day of surgery. Patients were kept at least six hrs nil by mouth prior to surgery On arrival to the operation theatre Ringer's Lactate solution was started intravenously through 20 g intravenous cannula at the rate of 10 ml/kg/hr and intravenous (IV) injection of ondansetron 4 mg was administered. Then standard monitoring including a non-invasive blood pressure monitor, pulse oximeter, five lead ECG nasal oxygen was applied and a wedge was kept under the right buttock. Foetal heart rate was confirmed by Doppler and the patient was allowed to rest for 5 minutes. The baseline systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) and pulse rate were noted. Afterwards the patient was given left lateral position and spinal anaesthesia was performed under all aseptic precautions with 25-gauge Quincke’s spinal needle in L3-4 / L4-5 space, 2.2 cc of heavy bupivacaine 0.5% was used to achieve spinal sensory level till T4. Patients were then immediately turned supine and wedges were kept under the right buttock. Patients with a failed spinal block, or inadequate level of spinal cord were excluded from the study. One of our colleagues who was not directly involved in the study prepared the drugs according to the randomization group. Thus he / she prepared two syringes, one 50 cc for infusion, and one 10 cc for bolus for each patient. In group A 50 cc syringe had phenylephrine 100 mcg /cc and 10 cc syringe had NS. In group B 50 cc syringe had NS and 10 cc syringe had phenylephrine 100 mcg/cc. The investigator who was the observer documented the haemodynamic readings and was unaware of the drug being given and thus double blinding was achieved. Protocol was to start infusion of study drug via syringe pump (Smith Medical) which was attached to the IV line via three way stopcock just after the placement of SAB at the rate of 1 cc/ min for three minutes. Then record SBP at 1 minute interval till delivery of the baby and anytime SBP was above baseline the infusion was to stop. Anytime BP falls below the baseline 1 cc bolus from 10 cc syringe was to be administered. Group A patients received prophylactic phenylephrine infusion 100 mcg/min and Group B patients received bolus phenylephrine to treat hypotension. In both groups, heart rate was managed by intravenous injection Atropine (0.6 mg) whenever the heart rate went below 50 beats/min if it was associated with hypotension. Post-delivery, APGAR score of the baby at 1minute, 5 minutes and umbilical arterial pH were measured. Statistical analysis: Sample size was calculated on the basis of a previous study by Warwick D. and Ngan Kee13 where the magnitude of hypotension was 21% in bolus group and 4% in the infusion group. Inserting these values in the following formula we got n=64, to adjust any fallouts we included. Sample Size n = (1.96)2 x P x Q / L2 P= proportion =21% Q = 100-P = 79% L = absolute error =10% α = 0.05% β= 80% All the data is organised in MS-Excel (2016) and analysed in R studio software (version 1.2.5001). Continuous variables are represented using mean and standard deviation. Mann Whitney U test, Chi square test and Fisher exact test are used to find the significant difference between the groups. The statistical significance of the difference between the groups is based on the ‘p’ value. A ‘p’ value of < 0.05 is considered as statistically significant. ‘P’ < 0.01 is considered as highly significant. ‘P’ >0.05 considered as not significant. OBSERVATIONS AND RESULTSTable 1 is the demographic observations and baseline haemodynamic characteristics and it shows no significant difference in both the groups, thus both groups were comparable. In Group A and B the mean systolic blood pressure (SBP) ranged between 101.83– 129.89 mm Hg and 99.54–117.03 mm Hg respectively (Figure 1). Though the baseline SBP of both the groups were comparable, significantly higher mean SBP (p < 0.01) was observed in Group A at 3-8 min after administration of infusion. In group A and B the mean diastolic blood pressure (DBP) ranged between 66.91-75.66 mm Hg and 58.09-74 mm Hg respectively (Figure 2). Baseline DBP of both groups were comparable. However, mean DBP was significantly high in Group A at 3-8 min post administration of infusion (P<0.05). The mean arterial pressure (MAP) in Group A and B ranged between 83.8–93.86 mm Hg and 71.54–88.83 mm Hg respectively (Figure 3). Baseline MAP of both the groups were comparable. However, in Group A MAP was significantly higher than group B at 3-8 min post administration of infusion (P<0.01). The mean heart rate in Group A and B ranged between 67.53-91.61 beats/minutes and 68.26-94.54 beats/minutes respectively (Figure 4). Basal heart rate of both groups was comparable. Comparatively, the mean heart rate at 3-5 minutes was significantly less in group A (P<0.01). However, no significant difference in heart rate was observed between the groups after 5 minutes. Neonatal outcomes with respect to umbilical arterial pH and APGAR scores are given in table 2. Though there was no foetal acidosis (pH < 7.2) in both groups, pH of group A babies was significantly higher than that of group B (p<0.01) indicating a good foetal profile with prophylactic infusion. Clinical evaluation by APGAR at 1 and 5 min showed no statistical difference in both the groups.
Table 1: Demographic profile and baseline haemodynamics of patients in both groups
Table 2: Neonatal outcomes
*P significant at <0.05 Flow chart: COSORT 2010: flow diagram for study design
Figure 1: Trends of SB Figure 2: Trends of DBP Figure 3: Trends of mean arterial blood pressure Figure 4: Trends of heart rate
DISCUSSION Though maternal blood volume in near term pregnant females is 40-50% more than in non-pregnant state the incidence of maternal hypotension after administration of SA pproaches 70%.1 Due to absence of autoregulation in placental circulation placental blood flow is largely dependent on maternal blood pressure. Thus maternal hypotension after SA leads to foetal acidosis and bradycardia.13,14.15 Untreated maternal hypotension is detrimental to mother and fetus. Researchers are yet to find the ideal technique or drug to prevent maternal hypotension. Reduction in central venous pressure due to aortocaval compression and marked reduction in systemic vascular resistance which follows sympathetic block after SA are the main factors to cause maternal hypotension. This is the reason fluid preloading and coloading has limited efficacy in preventing hypotension.16 Maintaining systemic vascular resistance, venous tone in splanchnic vasculature and venous capacitance are likely to be the key factors in preventing maternal hypotension. This emphasizes the role of vasopressors like ephedrine, mephentermine, metaraminol, phenylephrine in preventing hypotension as a physiological antidote. Although phenylephrine seems to have greater efficacy, its optimal dosage and method of administration is not yet standardized. Even though administration of boluses is a much simpler method, the continuous infusions have gained huge interest by showing promising results.10,17,18,19 The demographics of the participants were comparable in both the groups. The group that received prophylactic phenylephrine infusion showed less occurrence of hypotension as the mean SBP, DBP and MAP were comparatively high. Similar results were obtained by Choudhary et al.20 where researchers used either infusion or bolus phenylephrine to treat maternal hypotension and observed that the bolus group had 88% incidence of hypotension whereas the infusion group had just 4%. This is in agreement with Neves et al. where they noted that the incidence of hypotension was 85% in the treatment group versus 17% in the prophylactic infusion group.21 This reflects that a more stable management of blood pressure can be achieved by phenylephrine infusion due to stimulation of postsynaptic α receptors by it resulting in intense arterial and peripheral venoconstriction leading to rise in blood pressure. Heart rate lowered immediately post administration of prophylactic phenylephrine infusion, and it was significantly low in the infusion group upto 5 minutes. This can be attributed to baroreceptor reflex mediated bradycardia for which no treatment was required as blood pressure was more than baseline. However, heart rate at 6-8 minutes was not significantly different between the two groups as by that time blood pressure was stabilised in both the groups either by bolus or infusion. Despite periods of maternal hypotension in group B and decreased heart rate in group A, no significant difference in APGAR score was observed between both the groups similar results were observed in other studies.3,20 Although there was significant difference in uterine arterial pH among both the groups, foetal acidosis (pH <7.2) was not observed when phenylephrine was used either as prophylactic or therapeutic drug to treat hypotension in mothers. In group A prophylactic infusion resulted in higher fetal umbilical pH values (7.32) than in group B (7.29) This presumably could be due to maintenance of maternal blood pressure and uteroplacental blood flow until delivery. This also substantiates that the higher doses of phenylephrine used in prophylaxis has no detrimental effect on the fetus like in other studies.22,23 Limitations of the study include the small study population and the absence of a control group. Also there was no direct monitoring of cardiac output and stroke volume. However, a similar study with a large study population along with a control group would provide better insight on the effectiveness of phenylephrine in controlling hypotension in the patients undergoing CS under spinal anaesthesia. CONCLUSIONProphylactic infusion of phenylephrine is an effective measure in preventing hypotension and is also associated with better fetal outcome without adverse effects in women undergoing CS under SA. Declaration of patient consent. The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient (s) has / have given his /her /their consent for his /her /their clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed. REFERENCES
Policy for Articles with Open Access
|
|
 Home
Home