|
Table of Content - Volume 20 Issue 3 - December 2021
Comparative study of postoperative pain using an intraoperatively placed epidural catheter after major lumbar spine surgery
Hiral Virani1, Chetna Jadeja2*, Khyati Vaghela3, Swathy M4
1Senior Resident, 2Associate Professor, 3Assistant Professor, 4IIIrd Year Resident, Department of Anesthesiology, P.D.U. Government Medical College, Hospital Chowk, Jamnagar Road, Rajkot, INDIA. Email: hiral.virani20@gmail.com
Abstract Background: Spine surgeries are generally associated with intense pain in postoperative period. Adequate pain management may improve functional outcome, early ambulation, early discharge and preventing the development of chronic pain. Among various method of postoperative analgesia, continuous epidural analgesic infusion has less side effect and better patient compliance. This study was done to compare the effect of analgesia with epidural infusion of ropivacaine and tramadol in postoperative spine surgery. Method: After approval from ethical committee, fifty patients undergoing spine surgery, of either sex, aged 15 to 65years, American society of anesthesiologist physical status I, II, III were randomly allotted in group R and group T of 25 patients each. A multi hole epidural catheter was placed by the orthopaedic surgeon under direct vision when epidural space was opened during surgery. Analgesic infusion was given as below Group R - Ropivacaine 0.10% 10 ml bolus + 5 ml/hr for 24 hrs Group T- Tramadol 10 ml bolus (2 mg /ml) +0.2 mg/kg/hr for 24 hrs Result: There was no significance difference in VAS score between group T and group R at any hour postoperatively. Need of rescue analgesia in group R was more than group T but difference was not statistically significant. Hemodynemics and complications were comparable in both groups. Conclusion: Both Tramadol and Ropivacaine are equally effective for postoperative analgesia after major lumbar spine surgeries via continuous epidural infusion, without any major complications. Key words: Epidural infusion, postoperative analgesia, ropivacaine and tramadol, spine surgery.
INTRODUCTION Relieving post-operative pain of spine surgeries have become an indispensable component in anaesthesiology. Various methods have been tried for the management of post-operative analgesia in spine surgeries out of which epidural techniques are becoming most promising.1 Patients undergoing spine surgery experience severe pain in the postoperative period, which may increase morbidity and incidence of complications and prolong postoperative rehabilitation. In addition, postoperative pain itself is a risk factor for development of chronic pain syndromes.2,3 Postoperative pain therapy mainly exists in application of oral or intravenous opioids in combination with non-steroidal anti-inflammatory drugs but it often results in insufficient pain control and side effects such as respiratory depression, nausea, and vomiting. Epidural anesthesia and analgesia have been shown to be superior to intravenous analgesia with respect to pain quality, incidence of side effects pulmonary, cardiac and gastrointestinal dysfunction.4,5 Turner et al.6 showed in an observational study that epidural catheters placed intra operatively by the surgeon followed by infusion of local anesthetics with or without opioids were capable of providing good analgesia after posterior spinal fusion. Apart from dislocation of catheter, the placement of an epidural catheter in a recently operated area in the vertebral column with epidural application of local anesthetics may include the problem of unpredictable absorption of the drug and motor blockade. Although few studies of tramadol and ropivacaine are there, no study was done comparing effectiveness for postoperative analgesia between tramadol and any local anaesthetic as continuous epidural infusion. So we conducted this comparative study to evaluate postoperative analgesia and safety between Tramadol and Ropivacaine via intra -operatively placed epidural catheter after major lumbar spine surgery.
MATERIAL AND METHOD After approval from Institutional Ethics Committee, prospective, randomized double blind comparative study was carried out during 2017-2019 on 50 patients undergoing major lumber spine surgery of either sex, aged 15 to 65years, American society of anesthesiologist physical status I, II, III were randomly allotted in group R and group T of 25 patients each. It was comparative study between two groups using intra operatively placed epidural catheter using epidural ropivacaine and epidural tramadol for post op analgesia. Thorough pre anaesthetic checkup was done. Written informed consent was taken from each patients. Group R - Ropivacaine 0.10% 10 ml bolus + 5 ml/hr for 24 hrs. Group T-Tramadol 10 ml bolus (2 mg /ml) +0.2 mg/kg/hr for 24 hrs Exclusion criteria: History of allergy against local anesthetics. American Society of Anesthesiologists physical status class greater than III. infection in the area of the operation. postoperatively need for artificial ventilation for more than 2 h. operation of the cervical or thoracic spine. spinal metastasis. pre-existing pain symptoms apart from back pain. On the day before surgery, patients were examined with respect to hemodynamic variables. Patients were kept nil per oral 6-8 hour pre operatively, on the day of surgery, venous line inserted in the right or left forearm, and infusion of 500 ml Ringer’s solution (lactated) started. Operation performed in the prone position. In the operation theatre, all non-invasive monitoring devices (non-invasive blood pressure (NIBP), electrocardiograph leads, pulse oxymeter) were attached and the baseline cardiorespiratory parameters recorded. At the end of the posterior surgical procedure, a multi hole epidural catheter was placed by the orthopedic surgeon under direct vision when epidural space was opened during surgery. The catheter then tunnelled through the subcutaneous tissue, and the intact skin and secured with a single surgical knot. Catheters were always placed in the middle of the operation field and introduced 3 cm into the epidural space. After closure of the subcutaneous tissue 10-ml bolus of the respective study solution was infused through the epidural catheter after that an elastometric infusion pump (easy pump) connected to the epidural catheter. The study medication prepared by an independent anaesthesiologist who was not a part of study. After closing and dressing the surgical wound, patient made supine from prone surgical position and extubated after adequate reversal. Patients were shifted to post-operative room and monitored after administering the drug, the following parameters were noted by the independent observer. (1) The pain score, by using VAS at 0 hr., 6hr ,12hr, 18hr, 24hr, (2) Monitoring of vital parameters such as NIBP, pulse rate, respiratory rate every 30 min. (3) Side-effects such as nausea, vomiting, respiratory depression, motor blockade (Bromage scale>1), shivering and hypotension and (4) requirement for IV rescue analgesics (injection diclofenac 75mg diluted to 10ml). Syringes and pump of both study group appeared identical. A closed envelope with information about the patient’s study medication added in the patient’s record for emergency cases. All persons involved in the study were blind to the study medication. In postoperative period, the primary parameter to observe was postoperative pain at rest, in term of VAS score postoperatively. Secondary parameter was the amount of administered IV rescue analgesic in the same period. All patients have the option of receiving additional parenteral analgesia on request. Postoperative pain scores were recorded using a 0–10 cm visual analogue scale (VAS), on which a score of zero indicates no pain and a score of 10 indicates the worst conceivable pain. Inj. Diclofenac sodium 75mg diluted to 10 ml was used as rescue analgesic, routinely used at our center. Following parameters were observed: Pain score, sedation and respiratory rate. Blood pressure and heart rate. The patient’s ability to raise a straight leg. Pain at rest and on activity. Motor block-weakness of lower limb. Nausea. Itching. Urinary retention/incontinence. Headache. catheter related complication. Statistical analysis From previous data it is known that approximately 25% of the patients undergoing major lumbar spine surgery had a VAS score greater than 6 at rest. A clinical improvement of the pain scores in the group should include a 30% reduction of pain scores to be considered significant. Subsequently, a power analysis showed that a sample size of 21 patients per group was sufficient to have an 80% power at the 95% significance level. A sample size of 50 patients was obtained to overcome any potential dropouts. Demographic data are presented as medians and interquartile range, and rescue diclofenac consumption was assessed using the Mann-Whitney U test. OBSERVATIONS AND RESULT All continuous variables are presented as mean ± S.D and categorical variables are presented as absolute numbers and percentage. For all statistical test p value < 0.05 was statistically significant.
Table 1: Demographic profile of both groups
Demographic data were analysed by Mann Whitney U test. So, demographic data were comparable between two groups without significant difference.
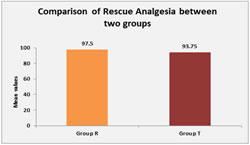
Graph 1 Graph 2 Mean VAS score was analysed by student’s unpaired t test. There was no significant difference in VAS score between group T and group R at any hr postoperatively. (p>0.05) Need of rescue analgesia analysed by Mann Whitney U test. Need of rescue analgesia in group R was more than group T but difference was not statistically significant. (p>0.05)
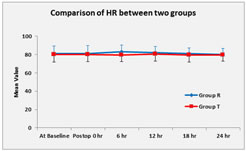
Graph 3 Graph 4 Mean heart rate was analysed by student’s unpaired t test. Graph 3 shows there was no significant difference in heart rate between two groups at any time postoperatively. (p>0.05) MBP was analysed by student’s unpaired t test. MBP was significantly lower in group R compare to group T at 6hr and 12hr postoperatively but no significant difference in MBP between two groups at 0hr, 18hr and 24hr postoperatively.
TABLE 2: Comparison of complications between two groups
complications were analysed by chi square test. Table 2 shows that complications between two groups were comparable without significant difference. (p>0.05)
DISCUSSION Postoperative pain therapy mainly exists in application of oral or intravenous opioids in combination with nonsteroidal anti-inflammatory drugs, but it often results in insufficient pain control and side effects such as respiratory depression, nausea, and vomiting. Epidural anesthesia and analgesia have been shown to be superior to intravenous analgesia with respect to pain quality, incidence of side effects, and pulmonary, cardiac, and gastrointestinal dysfunction. There are many studies of epidural ropivacaine.7,8,9. Epidural Ropivacaine may occasionally cause motor weakness of lower limb. In pilot study 0.125% ropivacaine was used but it developes motor weakness, this makes assessment of motor function by surgeons difficult. Tramadol if given epidurally have good analgesic effect without any motor effect. We gave Tramadol via epidural infusion 10mg per hour for 24hour so 240mg in 24 hour. Taking patient’s weight as 50 kg average, 4.8mg per kg Tramadol was given. This much dose of Tramadol have good analgesic effect without any significant major side effects. Vijayan R. et al. (April 1993)10 Studied the efficacy of epidurally administered tramadol hydrochloride, a weak centrally acting analgesic. In this study they used epidural tramadol maximum up to 400mg in 24 hour, it was very high dose compare to our study. In our study mean VAS score was less in group R compare to group T till 6 hr postoperatively. (Group R: 3.06± 0.74 vs group T: is 3.20± 1.04). But mean VAS score was comparatively lower in group T compare to group R, 6 hr after postoperatively till 24 hr. (group R: 1.74 ± 0.86 vs group T 1.56 ± 0.71). But result was statistically not significant at any time till 24hr post operatively. Although clinically patients in group R had less pain score initially up to 6 hr and then patients in group T had less pain score up to 24 hr but the difference was not statistically significant. This may be because Tramadol takes time to act and then accumulate over time. Md. Arshad Imam et al.11 studied 40 adult cases ranging in age from 20 to 60 years with ASA Grade I and II, presenting for elective gynecological surgery. Cases were randomly allocated into two groups containing 20 cases each. Cases in Group B received 10ml of 0.25% Bupivacaine and those in Group T received Tramadol 100mg in 10ml of normal saline. As results they found that Cases in Group T receiving epidural Tramadol had significant lower pain score on VAS as well as during 24 hours of observation. These cases also had significantly longer dosage intervals compared to Group B cases receiving Bupivacaine. In our study need of rescue analgesia was more in group R (mean 97.5%) compare to group T (mean 93.75%) but this difference is not statistically significant. Pulse rate between group R and group T, neither group R nor group T shows significant bradycardia or hypotension postoperatively. Shilpashri AM1 et al.12 a comparative study in 50 patients of age 18-60 years with American Society of Anesthesiologists grade I and II, undergoing elective abdominal and lower limb surgeries were randomly allotted to each of the 2 groups. Group BT received 0.25% bupivacaine + tramadol (1 mg/kg) and group RT received 0.25% ropivacaine + tramadol (1 mg/kg) epidurally. Patients were monitored for onset, duration and quality of analgesia, cardiorespiratory stability and for any side effects or motor blockade. In group BT, the mean pulse rate was 82.42 ± 14.41 beats/min at 0 min. There was fall in pulse rate starting from 76.96 ± 14.15 beats/min at 5 min to 72.42 ± 11.01 beats/min at 1 hour and then gradually increased to 78.88 ± 11.88 beats/min at 12 hours. In group RT the mean pulse rate was 81.08 ± 11.46 beats/min at 0 mins, with no significant fall in pulse rate. Compared to group BT, pulse rate in group RT was more stable. In our study there was no significant difference in pulse rate in both the group and pulse rate was stable in both group postoperatively. In group R 2 out of 25 cases (8%) developed hypotension and 92% cases have no any side effect. In 2 patients with hypotension SBP was reduced below 20% of baseline pre op SBP but it was not below 90 mmhg so no any vasopressure required for that. In group T 3 out of 25 cases (12%) developed nausea and 88% cases have no any side effect. Out of these three patients one patient had severe nausea (grade 2) and treated with ondansatron 4mg i.v. other two had only mild nausea (grade-1).
CONCLUSION From our study it can be concluded that Both Tramadol and Ropivacaine epidural infusion are equally effective for postoperative analgesia and have excellent safety profile. Side effects being minor and infrequent. Tramadol has side effect of nausea – vomiting while Ropivacaine has more propensity for hypotension. Ropivacaine at 0.1% does not causes motor weakness. So, both Tramadol and Ropivacaine are equally effective for postoperative analgesia after major lumbar spine surgery via continuous epidural infusion, without any major complications.
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home