|
Table of Content - Volume 21 Issue 2 - February 2022
Anaesthesia management of a patient posted for phylloid tumor excision with muscular dystrophy: A case report
Sneha Khobragade1*, Alok Kumar Swain2, Anshika Gier3
1,2,3Department of Anaesthesia, MMI Narayana Superspeciality Hospital, Raipur, Chhattisgarh, INDIA. Email: sneha88.sk@gmail.com
Abstract Background: Muscular dystrophy is the rare genetic disease-causing muscular weakness. The purpose of this report is to describe the major anaesthetic challenges and its postoperative complications. Major concerns related to these patients are difficult airway, comorbid cardiac disease with conduction defects, reduce pulmonary capacity, increased risk of developing extreme hyperthermia, rhabdomyolysis and hyperkalemic cardiac arrest when exposed to halogenated inhalational anaesthetics and depolarizing muscle relaxants and prolonged recovery from non-depolarizing muscle relaxants. All these factors are associated with increase postoperative complications. Proper planning and execution can help these patients to undergo surgery with minimum complications.
INTRODUCTION Muscular dystrophies are a genetic group of rare neuromuscular diseases with compromised synthesis in contractile proteins causing progressive muscular weakness. There are many kinds of muscular dystrophy, each affecting specific muscle groups, with signs and symptoms appearing at different ages, and varying in severity. The incidence of these diseases varies around 1 case per every 10,000 to 100,000 live births.1,35,36 With advances in medicine, these patients are living longer and are presenting to the hospital for multitude of surgical procedures. As a result, it is important to understand the anesthetic management of these patients in the perioperative periods. We are presenting a case report of successful anaesthesia management of phylloid tumor with progressive muscular dystrophy posted for lumpectomy.
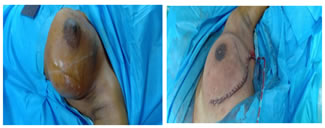
CASE REPORT A 21 years old female, weighing 40 kg with diagnosis of cystosarcoma phylloid tumor was posted for lumpectomy. She had 10*10 cm lobulated mass with variegated consistency almost involving whole left breast, confirmed on ultrasonography. She had progressively increasing bilateral upper limb and lower limb weakness since the age of 10 years and bed ridden since last 7 years. Mild wasting of bones was present, power in both upper limb and lower limb were 1/5 with normal tone of the muscle and no sensory involvement. Cardiovascular, respiratory systems and airway examination were normal. 2D ECHO showed akinetic mid posterior wall, hypokinetic mid lateral wall with fair LV function of 50-55 %. Her pulmonary function test and all other lab reports were within normal limits. Cardiologist reference was sort and as per his advice her cardiac enzymes were investigated with respect to changes in ECHO finding which were normal. Patient was planned for general anaesthesia with TIVA using IGEL for securing the airway and USG guided PEC II block with high-risk consent for requirement of postoperative ventilation. On the day of surgery, 8 hours NBM period was confirmed, patient was premedicated with 40 mg pantoprazole IV. After attaching the routine monitors, patient was induced with IV 1 mg midazolam, 100 mcg fentanyl and 100 mg propofol. Patient airway was secured with 3 No. IGEL. Ryles tube was passed through the IGEL to avoid gaseous distension of stomach. Under all aseptic precaution, US guided PECS – II block with 30 ml 0.2 % Ropivacaine + 1microgram/kg Dexmedetomidine was given. Out of this 30 ml, 10 ml was given for PECS – I and 20ml was given for PECS – II block in 5 ml of increment after negative aspiration of blood. Intraoperative monitoring included electrocardiography (ECG), non-invasive blood pressure, pulse oximetry, capnography and temperature. Anaesthesia was maintained with 70% nitrous oxide-oxygen-propofol and fentanyl infusion. As a part of multimodal analgesia, 1 gm paracetamol was given intravenously. Patient was left on spontaneous ventilation, ETCO2 was between 30 and 40 mm Hg. The intraoperative period was unremarkable with no abnormality in cardiac rhythm, ETCO2, body temperature and urine output. Propofol- fentanyl infusion was stopped 15 minutes prior to extubation. The total duration of the procedure was 2.5 hours. Patient was extubated when she was fully awake with good respiratory efforts. The patient was observed in post of recovery unit for signs of respiratory distress. She was comfortable, stable with no postoperative pain.27 She was shifted to room after 2 hours. There was no postoperative complications and was discharged from hospital on postoperative day 4. Figure 1: Preoperative and postoperative pictures
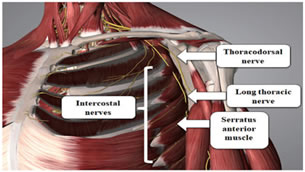
DISCUSSION The medical and surgical management of patients with muscular dystrophy can be challenging and fraught with complications (1, 2). For these reasons, coordination of the pre- and post-operative plans for care should be made 1-4 weeks in advance of elective procedures using a multi-disciplinary medical team. Specific perioperative concerns related to these patients are difficult airway, comorbid cardiac disease with conduction defects, reduce pulmonary capacity, increased risk of developing extreme hyperthermia, rhabdomyolysis and hyperkalemic cardiac arrest when exposed to halogenated inhalational anaesthetics and depolarizing muscle relaxants and prolonged recovery from non depolarizing muscle relaxants. All these factors are associated with increase postoperative complications. Cardiac disease is the common cause of death in patients with muscular dystrophy, with 10–20% of individuals are dying of cardiac failure. Dilated cardiomyopathy primarily involves the left ventricle, and can lead to dyspnea and other symptoms of congestive heart failure. Conversely, right ventricular failure can result from respiratory failure and pulmonary hypertension. They are also at risk for ventricular arrhythmias.2,3 Involvement of cardiologist becomes crucial in perioperative care of these patients. Respiratory muscle weakness predispose to restrictive lung disease with concurrent dyspnea and ineffective cough.9 Sleep disordered breathing, which may include nocturnal hypoventilation, nocturnal hypoxemia and obstructed sleep apnea with associated alteration in respiratory drive and marked central sensitivity to respiratory depressant drugs.9 Pulmonary function tests in both supine decubitus and seating position should be evaluated. If FVC is below 50% of the expected level, they are at risk for post-operative atelectasis and pneumonia as well as respiratory insufficiency requiring prolonged ventilation.1,8 There are weak evidences suggesting the possibility of a difficult airway must be considered,5,6 they present with Macroglossia. Patients with advanced stage of disease present with delayed gastric emptying time, weakness of the oropharyngeal muscles including swallowing difficulty and possible elevated risk of aspiration. We didn’t find difficult airway on airway assessment. We planned to secure the airway with IGEL as it causes less stress response and the patient was apprehensive of being awake during the surgery. IGEL also help in draining gastric content as these patients are at risk for aspiration secondary to their dysphagia and altered gastric motility.7 TIVA is beneficial for these patients.1,3,11 Propofol is the most commonly used hypnotic for the induction and maintenance of anesthesia in patients.12 Opoids, etomidate and thiopental have been successfully used.13 Volatile anesthetic agents are not used in most case reports, as these may trigger myotony and crisis of rhabdomyolysis. Nitrous oxide is the only inhaled agent used in around 34% to 78% of these reports.11,18 Use of depolarizing muscle relaxant is avoided as it is associated with unpredictable response, rhabdomyolsis.9,14 and may lead to a difficult or impossible intubation secondary to exaggerated contracture, masseter spasm, and laryngospasm. Delayed onsets of action and prolonged effect have been seen after the use of commonly used non depolarizing neuromuscular blocking agents.15,16,17 So we decided to avoid both depolarizing and non depolarizing muscle relaxant. Good analgesia is a must as it reduces any respiratory complications. However, keep in mind that these patients are more sensitive to the effects of opiates (systemic and neuraxial) and therefore present a higher risk of respiratory depression, exacerbated gastrointestinal paresis, and increased risk of reflux, aspiration, and ventilation dysfunction. The use of intravenous or rectal paracetamol has been reported.19,20 regional epidural anesthesia, and ultrasound-guided plexus nerve blocks as well as para-vertebral blocks for thoracic procedures.21,22,23 Regional anaesthesia techniques as a part of multimodal analgesia are regarded as the safe and best choice to reduce acute post operative pain and incidence of chronic pain after breast surgery.24 Pectoral nerve block is a novel superficial and lesser invasive block that provides good analgesia during and after ambulatory breast surgeries and is devoid of adverse hemodynamic effects.25,26,27,28 PECS I block Lateral and medial pectoral nerve and PECS II block Long thoracic, thoracodorsal and intercostal nerves (T2-6) (figure 2). Figure 2: Nerves involved in the pectoral nerve block
Ultrasound guidance has helped in providing better visualization of structures and improved analgesic effects by clearly seeing the diffusion of drug in the desired planes but also reduced the risk of inadvertent complications associated with performing a block blindly.29 Ropivacaine is a long acting amide with properties of less lipophilicity and high stereoselectivity. Thus it gives a greater degree of motor sensory differentiation, which could be useful when motor blockade is undesirable and also a higher threshold for cardiac and neurotoxicity.30,31 Dexmedetomidine is a selective shorter acting alpha-2 receptor agonist with sedative, analgesic and anaesthetic sparing properties and also provides minimal respiratory depression, cardiac protection, neuroprotection and renoprotection.32,33 For the PECS II block (figure 3), the local anaesthetic was injected as two parts (the first and the second injections). The first injection was the same as PECS I block. For the PECS I block (or the first injection of PECS II block), the probe was set to the anterior chest to confirm the location of the inter-fascial plane between the pectoralis major and pectoralis minor muscles at the 3rd rib, and local anaesthetic 10mL was injected at the inter-fascial plane. For the second injection the probe was attached onto the anterior chest to confirm the location of the inter-fascial plane between the pectoralis minor and the serratus anterior muscles at the 4th rib on the anterior axillary line and local anaesthetic 20 mL was injected at the inter-fascial plane.34
Figure 3: US image showing drug distribution between the muscle layers
Patients with muscular dystrophy have a high risk of apnea and death following extubation, over the next 24 hours after surgery.2 For extubation, the recommendation is to do it with the patient awake (1). Regardless of the anesthetic agent used, patients with dystrophies have a high risk of complications including respiratory failure, rhabdomyolysis, arrhythmias, cardiac arrest, and reactions similar to malignant hyperthermia that need acute symptomatic treatment, but fail to resolve with dantrolene.21,22 Patients with dystrophies may be classified based on the perioperative risk, in accordance with MIRS (muscular impairment rating scale); the risk is intermediate in grade MIRS 3, and very high in grades 4 and 5 (Table 1).23
Table 1: MIRS (muscular impairment rating scale) to classify muscular involvement in muscular dystrophy
CONCLUSION Muscular dystrophy is the rare genetic disease not commonly encountered in day to day clinical practice. The purpose of this report is to describe the major anaesthetic challenges and its postoperative complications. Proper planning and execution can help these patients to undergo surgery with successful outcome. Regional blocks helps in decreasing the dose of anaesthetic drugs required in general anaesthesia and decreases postoperative pain and complications.
REFERENCES Piedad cecilia echeverry-marín , ángela maría bustamante-vega. Anesthetic implications of muscular dystrophies. Rev. Colomb. Anestesiol. Vol.46 no.3 bogotá july/sept. 2018
Policy for Articles with Open Access
|
|
 Home
Home