|
Table of Content - Volume 21 Issue 2 - February 2022
Comparative evaluation of intrathecal administration of preservative free ropivacaine alone and combination of preservative free ropivacaine with dexmedetomidine in patients undergoing lower limb surgeries - A randomized trial
Sonam Norbu1, Dandub Gialchhen2*, Manoj Panwar3
1Assistant Professor, 3Professor, Department of Anaesthesia, IGMC Shimla, Himachal Pradesh 171001, INDIA. 2Anaesthesiologist, Deen Dayal Upadhyay Hospital Shimla, Himachal Pradesh 171001, INDIA.
Abstract Background: Local anesthetics used in spinal anesthesia are quite safe but duration of analgesia is limited. Dexmedetomidine, an α2 adrenergic agonist is a useful adjuvant to local anesthetics. The aim of the study is to evaluate the effect of dexmedetomidine on onset and duration of intrathecal ropivacaine. Material and methods: Seventy-five American Society of Anesthesiologists (ASA) physical status Classes I and II patients aged 18–65 years, scheduled for elective lower limb surgery were assigned to three groups of 25 each. Group I (n=25) received 22.5mg (3ml of 0.75%) of preservative free ropivacaine + 0.5 ml normal saline. Group II (n=25) received 22.5 mg (3ml of 0.75%) of preservative free ropivacaine + dexmedetomidine 5 μg in 0.5ml of normal saline intrathecally. Group III (n=25) received 22.5mg (3ml of 0.75%) of preservative free ropivacaine + dexmedetomidine 10 μg in 0.5ml of normal saline. Results: There was a dose dependent significant decrease in onset of sensory (11.26±1.24 vs 9.41±1.28 vs 7.86±1.79 min) and motor block (20.64 ± 6.48 vs 298.80 ± 74.57 vs 9.96 ± 1.94 min). However, time to reach peak sensory level was comparable in the groups. The two-segment regression time and the regression time of motor block to reach bromage zero was significantly prolonged in dose dependent manner. Conclusion: Use of intrathecal 5 μg and 10 μg dexmedetomidine as an adjuvant seems to be safe and effective alternative to opioids and other adjuvants for long duration surgical procedures due to its profound intrathecal analgesic properties with minimal adverse effects. Key Word: Dexmedetomidine, Ropivacaine, Sub-arachnoid Block.
INTRODUCTION Lower limb surgeries can be performed under neuraoxial or general anesthesia, but neuroaxial block is the preferred over other anaesthetic technique. Spinal anaesthesia is most commonly used for lower abdominal and lower extremity surgeries, with advantages of rapid onset, predictable duration, minimal side effects and less post operative morbidity.1 Bupivacaine, an amino-amide is the most popular agent among the local anaesthetic agents to be used for subarachnoid block, however it has been associated with systemic toxicity when used in high concentration or accidentally injected intravenously.2 Ropivacaine is a long-acting, amide local anesthetic that is structurally related to bupivacaine. Ropivacaine is developed for the purpose of reducing potential toxicity and improving sensory and motor block profile. To improve the quality and to extend the duration of spinal block, presently various intrathecal adjuvants are available, but the search for ideal adjuvant is still going on. Dexmedetomidine, a highly selective α2 -adrenergic agonist and acts as an analgesic and sedative, and has a higher α2a to α1 binding affinity (1300:1) than clonidine (39:1).3 During spinal anaesthesia, dexmedetomidine is administered as an adjuvant to increase depth of block and increase the duration of block along with decrease in time of onset of block. It reduces systemic absorption and therefore prevents side effects. It also reduces opioids and inhalational anaesthetics requirements.4 Intrathecal α2- receptor agonists are found to have antinociceptive action for both somatic and visceral pain.5 The aim of the study is to evaluate the effect of dexmedetomidine as an adjuvant on onset and duration of intrathecal ropivacaine.
MATERIALS AND METHODS A randomized study was conducted on seventy-five American Society of Anesthesiologists (ASA) physical status Classes I and II patients aged 18–65years, scheduled for elective lower limb surgery. After Institutional Ethics Committee approval and informed consent, patients were randomized into three Groups I, II, and III. Patients of ASA physical status > II, patients having renal disorder, psychosis, uncooperative, peripheral neuropathy, demyelinating central nervous system disorder, scoliosis, allergic to dexmedetomidine or local anaesthetics and coagulopathy were excluded In the operation theater, electrocardiography, peripheral oxygen saturation, and noninvasive blood pressure (BP) were connected, and basal parameters were recorded. Intravenous (IV) access was obtained on the nondominant hand with 18-gauge cannula, and crystalloid infusion started. Oxygen was administered by the facemask. Under strict asepsis, spinal anesthesia was performed at L3–L4 interspace with a 25-gauge Quincke needle by a midline approach with the patient in sitting position. Group I (n=25) received 22.5mg (3ml of 0.75%) of preservative free ropivacaine + 0.5 ml normal saline. Group II (n=25) received 22.5 mg (3ml of 0.75%) of preservative free ropivacaine + dexmedetomidine 5 μg in 0.5ml of normal saline intrathecally. Group III (n=25) received 22.5mg (3ml of 0.75%) of preservative free ropivacaine + dexmedetomidine 10 μg in 0.5ml of normal saline. Medication used for spinal anaesthesia was prepared and administered by an anesthesiologist not involved in the collection of data. The completion of spinal injection was taken as the time zero for induction of anesthesia. Systolic arterial pressure , diastolic arterial pressure, mean arterial pressure, heart rate, peripheral oxygen saturation were monitored every three minutes for first 30 minutes followed by every 10 minutes for next 30 minutes and then every 20 minutes till 120 minutes. Sensory level was assessed by loss of sensation to pinprick in the midclavicular line bilaterally. Time to reach sensory level of T10 was taken as the time of onset of analgesia. During the tracking of the sensory block levels in patients, the maximum sensory block level, time to achieve maximum sensory block and 2-segment regression time of the sensory block was noted. Motor block was assessed according to modified Bromage scale6 (0: No motor block, 1: Inability to raise extended legs, 2: Inability to flex knees, and 3: Inability to flex ankle joints). Time taken to reach Bromage 3 was taken as the time of onset of motor block. Statistical analysis The data were analyzed using statistical package for social science (SPSS 21.0 evaluation version). Data was expressed as means and standard deviation (SD), medians and ranges, or numbers and percentages. For categorical covariates Chi-square test or Fisher’s exact test was used as appropriate, with P value reported at the 95% confidence interval (CI). Continuous covariates were compared using analysis of variance (ANOVA). If P value was significant, then Bonferroni test for post hoc analysis. RESULTS Seventy-five patients were randomly allocated to three groups of 25 patients. All the enrolled patients completed the study. Demographic data were comparable in the three groups (Table 1) Table 1
The onset of analgesia (time to reach T10 sensory level) was slower in Group I (11.26±1.24 min) compared to Group II (9.41±1.28 min) and III (7.86±1.79. min). Two segment regression from highest sensory level was faster in Group I (93.36±21.56) compared to group II (129.04±13.46) and group III (164.84±20.82). However, Time to reach peak sensory level was comparable in the three groups. The onset and the duration of motor block were significantly faster in group III compared to Group I and Group II (Table 2) Table 2
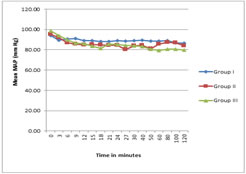
There was a gradual decrease in the mean arterial pressure (MAP) intraoperatively, but none of the patients had significant hypotension. Changes in MAP mean were comparable between three groups (Figure 1). Hypotension developed was manageable with Inj. Mephentermine 3mg. In Group I, 8% of patients required single dose of inj. Mephentermine. In Group II, 12% required single dose of inj. Mephentermine. In Group III, 8% of patients required single dose of inj. Mephentermine and 8% of the patients required two doses inj. Mephentermine (P = 0.626). Figure 1 Figure 2 Figure 1: Comparison of intraoperative MAP at different time intervals; Figure 2: Comparison of intraoperative HR at different time intervals There were no significant differences in perioperative HR. Bradycardia was not reported in any groups (Figure 2) Side effects No patients required atropine. Intra-operative or post-operative nausea or vomiting did not occur in any of the three groups of patients. The 2-week follow-up questionnaire did not show any new onset of back, buttock or leg pain or paresthesias.
DISCUSSION Spinal anaesthesia is being practiced safely and effectively for many years. Spinal anaesthesia is a proven and relatively safe method of regional anaesthesia in lower limb surgery. The pharmacological and physiological characteristics of local anaesthetic used in spinal anaesthesia should have rapid onset, rapid spread with deep penetration of nerves, low tissue and systemic toxicity and prolonged duration of action, so that a single dose rather than a continuous dose technique might be employed. These characters should not depend on the use of vasoconstrictive drug in local anaesthetic solution.7 All local anesthetic used in spinal anaesthesia has limited duration of action. To prolong the duration of action of local anesthetic, their various adjuvants are available. The addition of adjuvants in spinal anaesthesia help in reduction of local anaesthetic dose, thus provides more hemodynamic stability and reduces systemic toxicity of local anaesthetic, as well as prolonging their duration of action. However, additive like opioids can produce side effects such as nausea, vomiting, pruritus, and respiratory depression. Intrathecal α2 agonists potentiates the effects of local anesthetics and allow a decrease in dose without respiratory depression and hemodynamic instability. Dexmedetomidine is believed to act at the spinal and supraspinal receptors.8,9 Compared to its counterpart, clonidine, it has 8-fold greater affinity for α2 receptors. In this study, we evaluated the efficacy of ropivacaine alone or with 5ug dexmedetomidine or with 10ug dexmedetomidine in elective lower limb surgery. The results of our study show that supplementation of spinal ropivacaine with dexmedetomidine significantly prolonged both sensory and motor block in dose dependent manner compared with ropivacaine alone. Eid et al. demonstrated that dexmedetomidine 10 μg gave faster onset and longer duration of block as well as postoperative analgesia in comparison with 5μg intrathecal dexmedetomidine for lower limb surgeries.10 However, Naaz et al. found that at higher doses of 15 and 20 μg of dexmedetomidine produces significant hypotension and bradycardia with prolong duration of sensory and motor block in a dose-dependent manner.11 In a meta-analysis, when dexmedetomidine used intrathecally, it hastened the sensory block onset by 19%, prolonged motor block duration by 88%, and delayed the time of the first analgesic request by 127% compared with local anesthetic alone.12The duration of motor block as observed in our study was markedly prolonged (348.64 ±78.30 min) with 10ug intrathecal dexmedetomidine and with 5ug intrathecal dexmedetomidine (298.80±74.57) when compared to the duration of motor block of 273.3 ± 24.6 min in ropivacaine group. The onset of motor and sensory block was faster in dexmedetomidine group in dose dependent manner. Marhofer et al. demonstrated that dexmedetomidine hasten the onset time of motor block when added to local anesthetic for peripheral nerve block.13 However, time to achieve peak sensory level were comparable between the groups (p = 0.066). Al-Mustafa et al.14 compared the doses of dexmedetomidine 5 , 10 µg in isobaric bupivacaine 12.5mg (total volume:3 ml) with plain isobaric bupivacaine without premedication and found the effect to be dose dependent on the onset and regression of sensory and motor block with comparable sedation scores among three groups Dexmedetomidine has α2 antinociceptive action for both somatic and visceral pain.15 Mechanisms by which they prolong motor and sensory blocks of local anesthetics are not known. Local anesthetics act by sodium channel blockade. α2 adrenergic agonist binds to presynaptic C fibers and postsynaptic dorsal horn neurons. Hence, the analgesic effect may be due to the depression of release of C-fiber transmitters and hyperpolarization of postsynaptic dorsal horn neurons. Prolonged motor blockade might be caused by direct impairment of excitatory amino acids from the spinal interneurons.15 The hemodynamic profile was similar in all the three groups. The most significant side effects reported about the use of intrathecal a2 adrenoreceptor agonists are bradycardia and hypotension.16 Al-Ghanem et al.17 have reported the use of dexmedetomidine to be associated with a decrease in heart rate and blood pressure. In the present study, these side effects were not significant. However, the consumption of inj. Mephentermine was more in group III in comparison with group I and II but was insignificant. This study has some limitations. As all patients were either ASA physical status I or II, so results cannot be generalised to ASA physical status III and IV patients. Hence, further studies that compare the effect of intrathecal dexmedetomidine on the spinal ropivacaine with large sample size will be required. In conclusion, use of intrathecal 5 μg and 10 μg dexmedetomidine seems to be safe and effective alternative to opioids and other adjuvants for long duration surgical procedures due to its profound intrathecal analgesic properties with minimal adverse effects. However, prolonged duration of motor blockade with dexmedetomidine may be undesirable for short-term surgical procedures or ambulatory surgeries. In future, further large randomized studies are recommended to prove its safety and efficacy.
REFERENCES
Policy for Articles with Open Access
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Home
Home